1. High-Performance Asymmetric Supercapacitors of MnCo2O4 Nanofibers and N-Doped Reduced Graphene Oxide Aerogel
By: Pettong, Tanut; Iamprasertkun, Pawin; Krittayavathananon, Atiweena; et al.
ACS APPLIED MATERIALS & INTERFACES, 2016, 8, 34045-34053
DOI : https://pubs.acs.org/doi/10.1021/acsami.6b09440
Abstract: The working potential of symmetric supercapacitors is not so wide because one type of material used for the supercapacitor electrodes prefers either positive or negative charge to both charges. To address this problem, a novel asymmetrical supercapacitor (ASC) of battery-type MnCo2O4 nanofibers (NFs)//N-doped reduced graphene oxide aerogel (N-rGOAE) was fabricated in this work. The MnCo2O4 NFs at the positive electrode store the negative charges, i.e., solvated OH-, while the N-rGOAE at the negative electrode stores the positive charges, i.e., solvated K+. An as-fabricated aqueous-based MnCo2O4//N-rGOAE ASC device can provide a wide operating potential of 1.8 V and high energy density and power density at 54 W h kg-1 and 9851 W kg-1, respectively, with 85.2% capacity retention over 3000 cycles. To understand the charge storage reaction mechanism of the MnCo2O4, the synchrotron-based X-ray absorption spectroscopy (XAS) technique was also used to determine the oxidation states of Co and Mn at the MnCo2O4 electrode after being electrochemically tested. The oxidation number of Co is oxidized from +2.76 to +2.85 after charging and reduced back to +2.75 after discharging. On the other hand, the oxidation state of Mn is reduced from +3.62 to +3.44 after charging and oxidized to +3.58 after discharging. Understanding in the oxidation states of Co and Mn at the MnCo2O4 electrode here leads to the awareness of the uncertain charge storage mechanism of the spinel-type oxide materials. High-performance ASC here in this work may be practically used in high-power applications.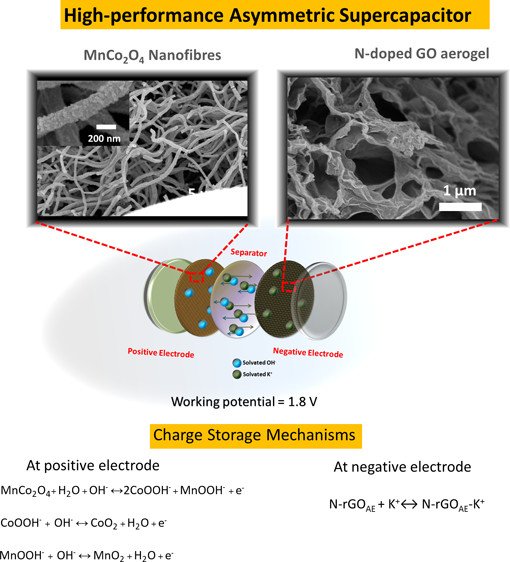

2. CO2 hydrogenation to methanol using Cu-Zn catalyst supported on reduced graphene oxide nanosheets
By: Deerattrakul, Varisara; Dittanet, Peerapan; Sawangphruk, Montree; et al.
Journal of CO2 Utilization, 2016, 16, 104-113
DOI : https://www.sciencedirect.com/science/article/pii/S221298201630155X?via%3Dihub
Abstract: Supported Cu-Zn/reduced graphene oxide (rGO) catalysts were synthesized via an incipient wetness impregnation and evaluated for a CO2 hydrogenation to methanol. The structure, surface reactivity, and adsorption properties were investigated extensively by FE-SEM, TEM, XRD, Raman, FT-IR, N2 sorption, TGA, TPR, and XPS techniques. The effects of Cu-Zn metal loading content and the reaction temperature were investigated toward the methanol production from the CO2 hydrogenation. It was found that supported rGO nanosheets could greatly enhance the catalytic performance and help the dispersion of bimetallic compounds Cu-Zn particles. The 10 wt%CuZn/rGO catalyst yielded the highest space time yield (STY) of 424 mgMeOH gcat−1 h−1 at a reaction temperature and pressure of 250 °C and 15 bar, respectively.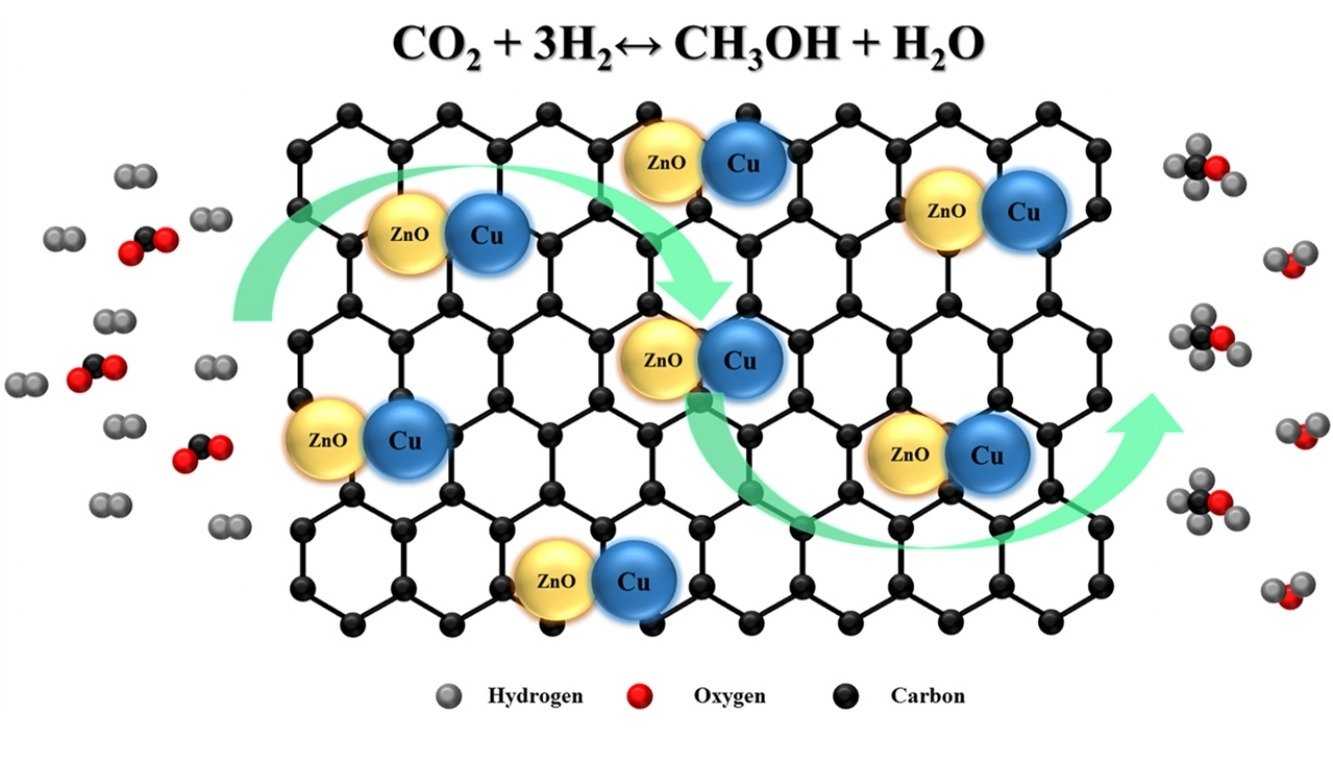

3. Charge storage mechanisms of manganese oxide nanosheets and N-doped reduced graphene oxide aerogel for high-performance asymmetric supercapacitors
By: Iamprasertkun, Pawin; Krittayavathananon, Atiweena; Seubsai, Anusorn; et al.
Scientific Reports, 2016, 6, 37560
DOI : https://www.nature.com/articles/srep37560
Abstract: Although manganese oxide- and graphene-based supercapacitors have been widely studied, their charge storage mechanisms are not yet fully investigated. In this work, we have studied the charge storage mechanisms of K-birnassite MnO2 nanosheets and N-doped reduced graphene oxide aerogel (N-rGOae) using an in situ X-ray absorption spectroscopy (XAS) and an electrochemical quart crystal microbalance (EQCM). The oxidation number of Mn at the MnO2 electrode is +3.01 at 0 V vs. SCE for the charging process and gets oxidized to +3.12 at +0.8 V vs. SCE and then reduced back to +3.01 at 0 V vs. SCE for the discharging process. The mass change of solvated ions, inserted to the layers of MnO2 during the charging process is 7.4 μg cm−2. Whilst, the mass change of the solvated ions at the N-rGOae electrode is 8.4 μg cm−2. An asymmetric supercapacitor of MnO2//N-rGOae (CR2016) provides a maximum specific capacitance of ca. 467 F g−1 at 1 A g−1, a maximum specific power of 39 kW kg−1 and a specific energy of 40 Wh kg−1 with a wide working potential of 1.6 V and 93.2% capacity retention after 7,500 cycles. The MnO2//N-rGOae supercapacitor may be practically used in high power and energy applications.
4. Enhancing the charge-storage performance of N-doped reduced graphene oxide aerogel supercapacitors by adsorption of the cationic electrolytes with single-stand deoxyribonucleic acid
By: Krittayavathananon, Atiweena; Iamprasertkun, Pawin; Sawangphruk, Montree
Carbon, 2016, 109,, 314-320
DOI : https://www.sciencedirect.com/science/article/pii/S000862231630673X?via%3Dihub
Abstract: Single-stand deoxyribonucleic acid/N-doped reduced graphene oxide aerogel (ssDNA/N-doped rGO) composites were synthesized and used as the electrode materials for supercapacitors. The mass change of the electrolytes adsorbed on the ssDNA/N-doped rGO electrode during a charge/discharge process determined by an electrochemical quartz crystal microbalance (EQCM) at a cathodic potential range (−0.5 to −0.1 V vs. Ag/AgCl) is 10-fold higher than that at an anodic potential range (−0.1–0.3 V vs. Ag/AgCl). This is because the ssDNA having negatively charged phosphate and nitrogen-containing nucleobase groups prefers cationic electrolytes to anionic ones. An as-fabricated supercapacitor of the ssDNA/N-doped rGO with a coin-cell size (CR2016) provides 644 F/g at 0.5 A/g, which is 1.3-fold higher than that of the N-doped rGO device. The specific power and specific energy of the as-fabricated ssDNA/N-doped rGO device are ca. 30 Wh/kg and 4500 W/kg, respectively with a capacity retention of 94.1% over 2500 cycles. Two as-fabricated supercapacitor cells connected in series can supply electricity to the red LED over 10 min. The composite materials in this work may also be used in other applications where the functional groups of ssDNA can play an important role to the adsorption of the cationic electrolytes.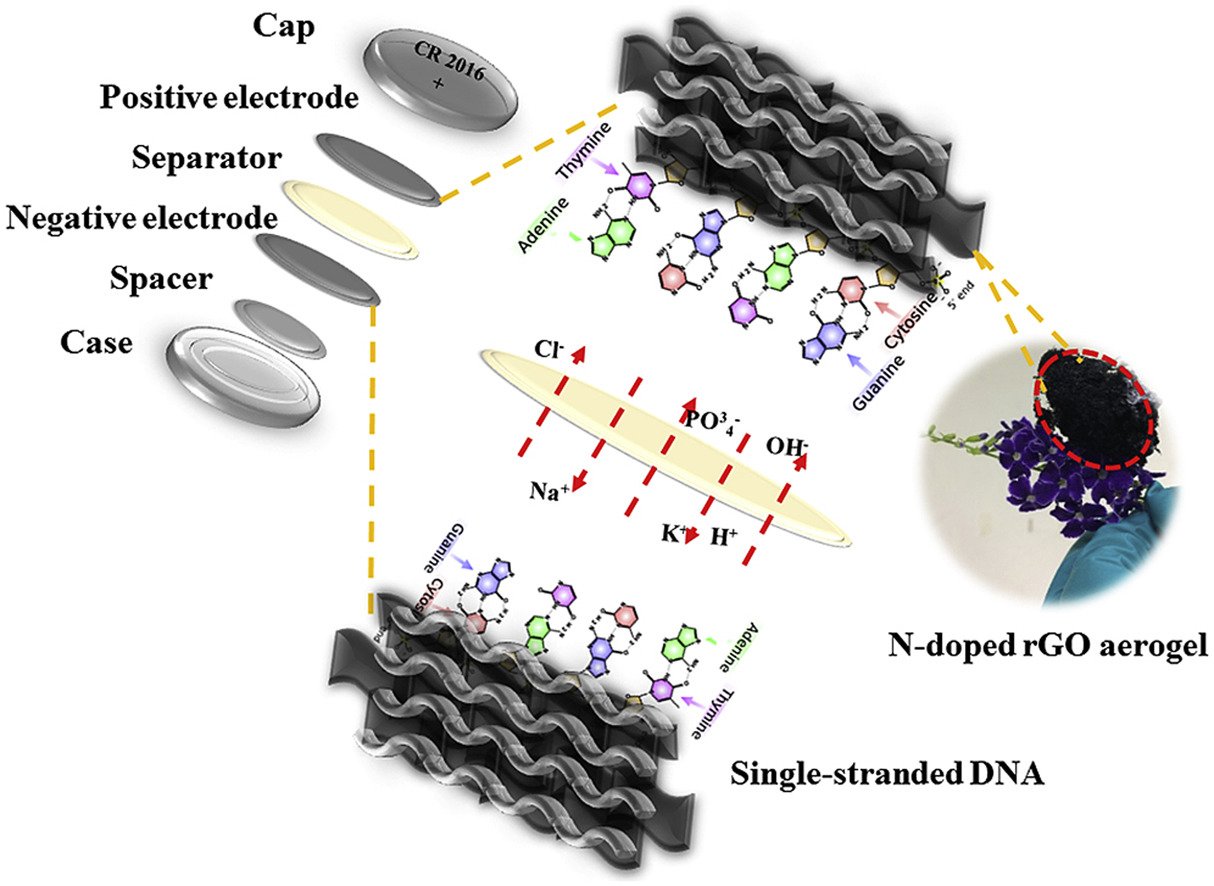

5. Electrocatalytic oxidation of ethylene glycol on palladium coated on 3D reduced graphene oxide aerogel paper in alkali media: Effects of carbon supports and hydrodynamic diffusion
By: Krittayavathananon, Atiweena; Sawangphruk, Montree
Electrochimica Acta, 2016, 212, 237-246
DOI: https://www.sciencedirect.com/science/article/pii/S0013468616314918?via%3Dihub
Abstract: To enhance Pd electro-catalysts in their electro-catalytic activity for C-C bond breaking and tolerance stability, Pd was electrodeposited on high-porosity 3D reduced graphene oxide (rGO) aerogel paper. For comparison, the Pd was also coated on other three different carbon material surfaces: bare carbon fiber paper (CFP), and CFP modified with either 2D graphene oxide (GO) and 2D rGO nanosheets. The Pd/3D rGO aerogel paper exhibits higher catalytic activity toward the electro-oxidation of ethylene glycol in alkaline media and higher excellent tolerance stability than other electrodes due to ultra-high porosity of the 3D rGO support leading to high active electrochemical surface area of the as-fabricated Pd catalyst electrode. In addition, the hydrodynamic diffusion of ethylene glycol to the cayalyst surface investigated in this work plays a major role to the catalytic activity of the as-prepared electrocatalyst. The Pd/3D rGO aerogel rotating disk electrode exhibits a high anodic current density of 267.8 mA cm−2 at 3000 rpm. This high-performance Pd/3D rGO aerogel may be practically used as the high-efficiency anode of direct ethylene glycol and other related alcohol fuel cells.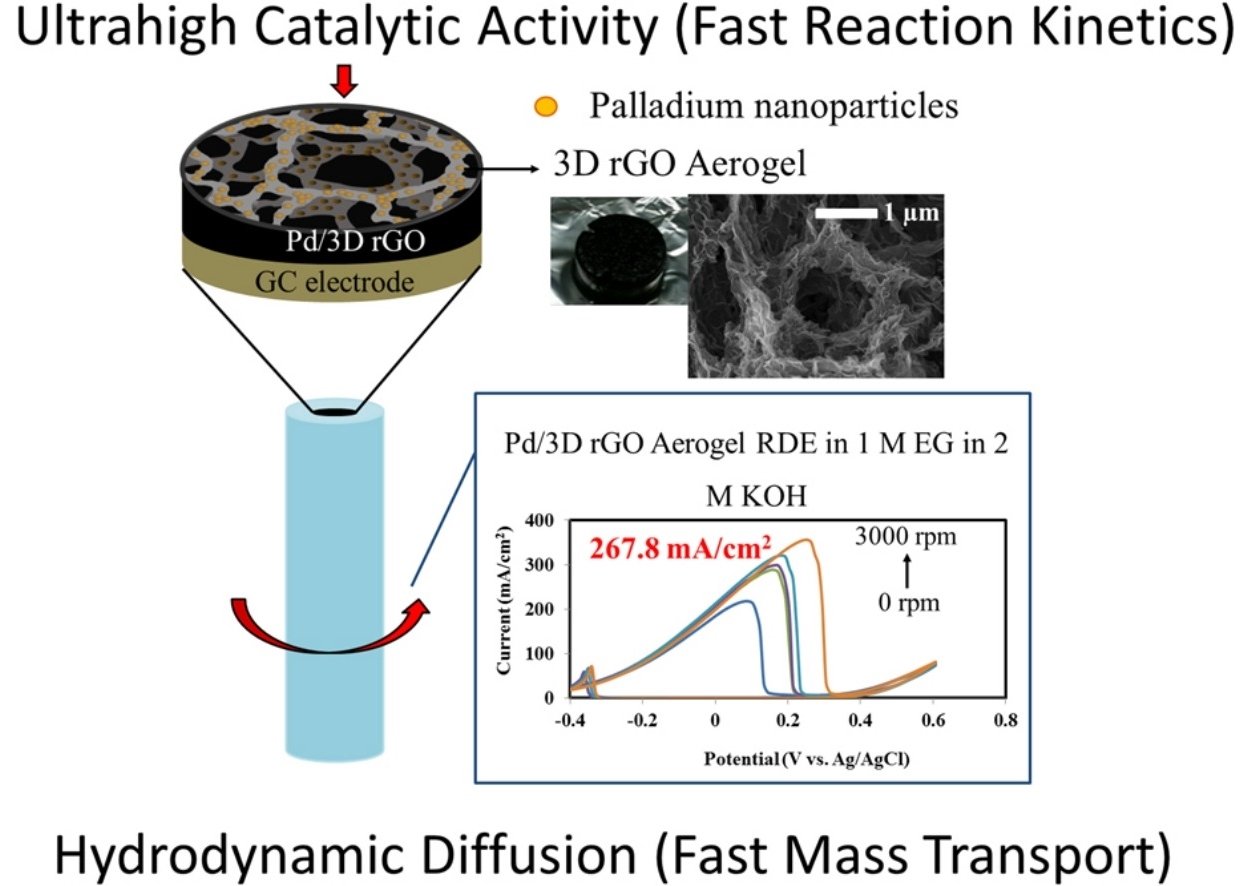

6. New Routes to Functionalize Carbon Black for Polypropylene Nanocomposites
By: Shepherd, Celine; Hadzifejzovic, Emina; Shkal, Fatma; et al.
Langmuir 2016, 32, 7917–7928
DOI : https://pubs.acs.org/doi/10.1021/acs.langmuir.6b02013
Abstract: Methods for chemical surface functionalization for carbon black (CB) nanoparticles were studied to produce (CB)/polypropylene (PP) nanocomposites with superior electrical and thermal properties. Nanoparticle dispersion is known to directly control the extent to which nanocomposites maximize the unique attributes of their nanoscale fillers. As a result, tailored nanoparticle surface chemistry is a widely utilized method to enhance the interfacial interactions between nanoparticles and polymer matrices, assisting improved filler dispersion. In this work, a rapid chemical functionalization approach using a number of diarylcarbene derivatives, followed by the azo-coupling of substituted diazonium salts, for the covalent introduction of selected functional groups to the CB surface, is reported. Characterization of the modified CB by XPS, TGA, CHN, and ATR-IR collectively confirmed surface functionalization, estimating surface grafting densities of the order of 1013 and 1014 molecules/cm2. Nanocomposites, synthesized by solvent mixing PP with pristine and modified CB, demonstrated macroscopic property changes as a result of the nanoparticle surface functionalization. Pronounced improvements were observed for PP nanocomposites prepared with a dodecyl-terminated diaryl functionalized CB, in which TEM analysis established improved nanofiller dispersion owing to the enhanced CB-PP interfacial interactions in the nanocomposite. Observed dielectric relaxation responses at 20 wt % loading and a reduced percolation threshold realized conductivities of 1.19 × 10–4 S cm–1 at 10 wt %, compared to 2.62 × 10–15 S cm–1 for pristine CB/PP nanocomposites at the same filler loading. In addition, thermal properties signify an increase in the number of nucleation sites by the raised degree of crystallinity as well as increased melting and crystallization temperatures.

7. N-doped reduced graphene oxide aerogel coated on carboxyl-modified carbon fiber paper for high-performance ionic-liquid supercapacitors
By: Iamprasertkun, Pawin; Krittayavathananon, Atiweena; Sawangphruk, Montree
Carbon, 2016, 102, 455-461
DOI : https://www.sciencedirect.com/science/article/pii/S0008622315305583?via%3Dihub
Abstract: Nitrogen-doped reduced graphene oxide aerogel (N-rGO aerogel) with high porosity and ionic conductivity were synthesized by a hydrothermal reduction of graphene oxide with hydrazine and following freezing-dry method. N-rGO aerogel was spray-coated on carboxyl-modified carbon fiber paper with a hydrophilic surface and used as the supercapacitor electrode. Not only can the N-rGO aerogel electrode accelerate the diffusion of the electrolyte but also it can store electronic charge via a surface redox reaction due to the N-containing groups. Among the electrolytes studied, the ionic liquid-based supercapacitor of the N-rGO aerogel provides a wide working potential of ca. 4.0 V and rather high specific capacitance of 764.53 F/g at 1 A/g with a capacity retention of 86% over 3000 charge–discharge cycles as well as maximum specific power and energy of 6525.56 W/kg and 245.00 Wh/kg, respectively. The device prototype fabricated in a single coin cell shape (CR2016) can supply electricity to red LED over 17 min.
8. Turning conductive carbon nanospheres into nanosheets for high-performance supercapacitors of MnO2 nanorods
By: Phattharasupakun, Nutthaphon; Wutthiprom, Juthaporn; Chiochan, Poramane; et al.
Chemical Communications, 2016, 52, 2585-2588
DOI : https://pubs.rsc.org/en/content/articlelanding/2016/cc/c5cc09648k
Abstract: Oxidized carbon nanosheets (OCNs), produced from black carbon nanospheres and used as a conductive additive in the supercapacitor electrodes of MnO2 nanorods, can significantly improve the charge-storage performance of the symmetric MnO2-nanorod supercapacitors with a maximum specific energy of 64 W h kg−1 and power of 3870 W kg−1. An optimum material composition of the supercapacitor electrode finely tuned is 60 : 30 : 10 wt% of MnO2 : OCN : PVDF, respectively. Interestingly, after 5000 charge/discharge cycles, the oxidation numbers of Mn at the positive and negative electrodes of the as-fabricated supercapacitor are +3.22 and +3.04, respectively.