1. A single energy conversion and storage cell of nickel-doped cobalt oxide under UV and visible light illumination
By: Kongsawatvoragul, Ketsuda; Kalasina, Saran; Phattharasupakun, Nutthaphon; et al.
Electrochimica Acta, 2019, 328, 135120
DOI: https://www.sciencedirect.com/science/article/pii/S0013468619319917?via%3Dihub
Abstract: The NixCo3-xO4, a p-type semiconductor, with the optical gap energies of ca. 2.1 and 3.5 eV generates an electron-hole pair via the photoelectric effect under light illumination. Although NixCo3-xO4 is widely investigated as a photocatalyst and energy storage electrode, the charge storage mechanism of NixCo3-xO4 under light illumination is not so clear. Herein, the electrochemical performance of NixCo3-xO4 material was investigated under three systems (dark, visible light and UV–visible light). During the photoexcitation, positive charge, holes of NixCo3-xO4 can effectively react with the electrolyte (OH−) to generate NiO(OH) and CoO(OH) since the holes can move through space by the transfer of electron to the vacancy and react with the hydroxide ions of the electrolyte solution. Interestingly, the specific capacity of NixCo3-xO4 (mAh g−1) increases by ca. 51.2% under visible light and ca. 59.9% under UV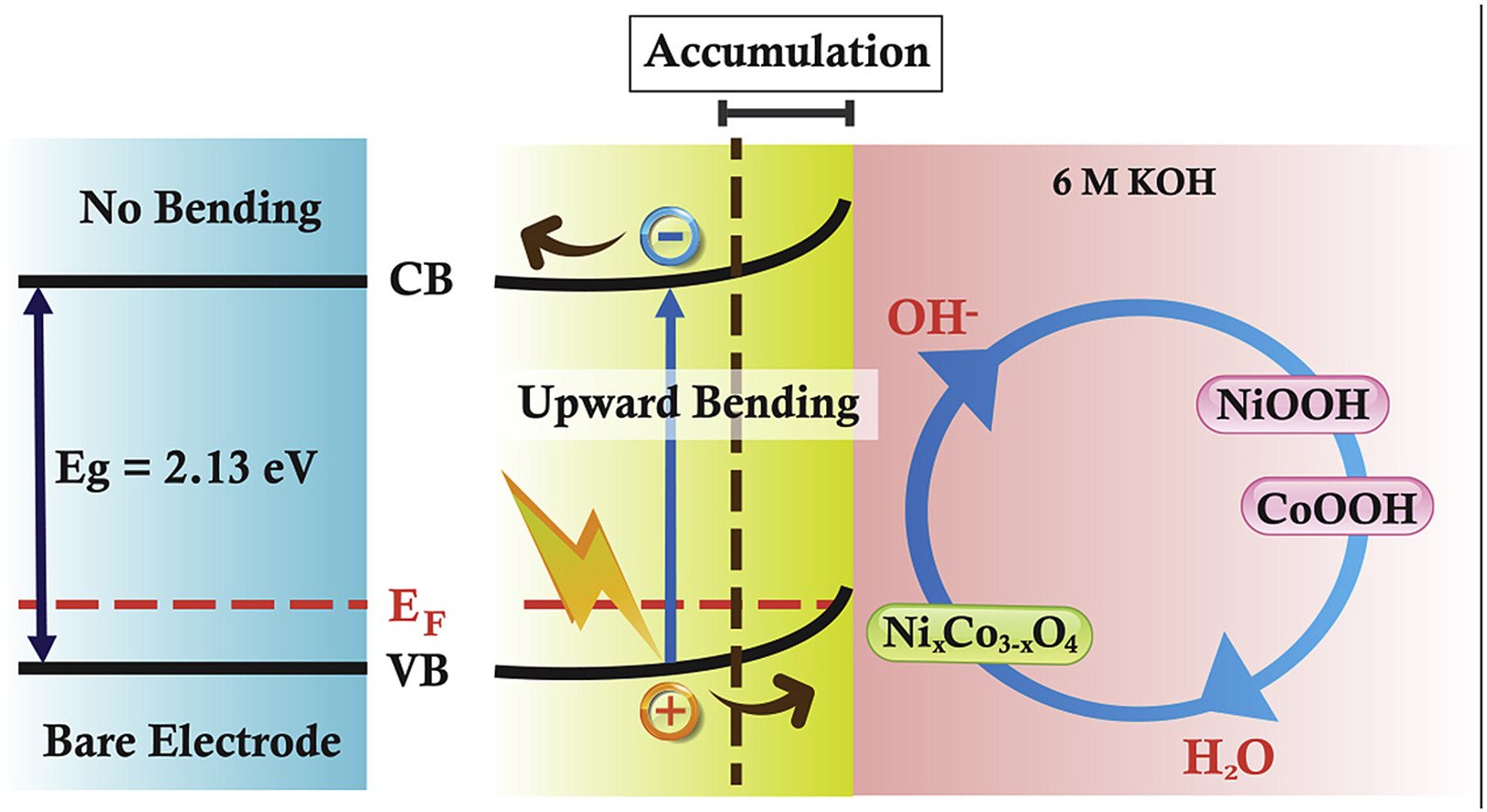

2. Heterogeneous structural defects to prompt charge shuttle in g-C3N4 plane for boosting visible-light photocatalytic activity
By: Yang, Chengwu; Xue, Zhe; Qin, Jiaqian; et al.
Applied Catalysis B: Environmental, 2019, 259, 18094
DOI: https://www.sciencedirect.com/science/article/pii/S0926337319308410?via%3Dihub
Abstract: Structural defect engineering toward g-C3N4 plane usually has great benefit on modulating electron structure and photocatalytic performance. Here, we report a porous g-C3N4 material with heterogeneous structural defects, oxygen atom replacing edge nitrogen and cyano group, obtained via a facile treatment method. The reconstructed material shows narrowing band gap, high light absorption and fast charge separation. Theoretical calculation discloses that the doped oxygen atom and the nearby atoms accept electrons as reduction site to produce hydrogen, while the undoped fraction and cyano group take the duty to oxidize water. The delocalization of reactive sites drives charge shuttle on the plane, limiting recombination of charge carriers. Consequently, the modified g-C3N4 shows excellent photocatalytic activity with apparent quantum efficiency of 8.41% under 420 nm wavelength, surpassing pure g-C3N4 and other reported materials with defect compilation. We think that this work provides a new avenue to understand the function of structural defect on prompt charge separation.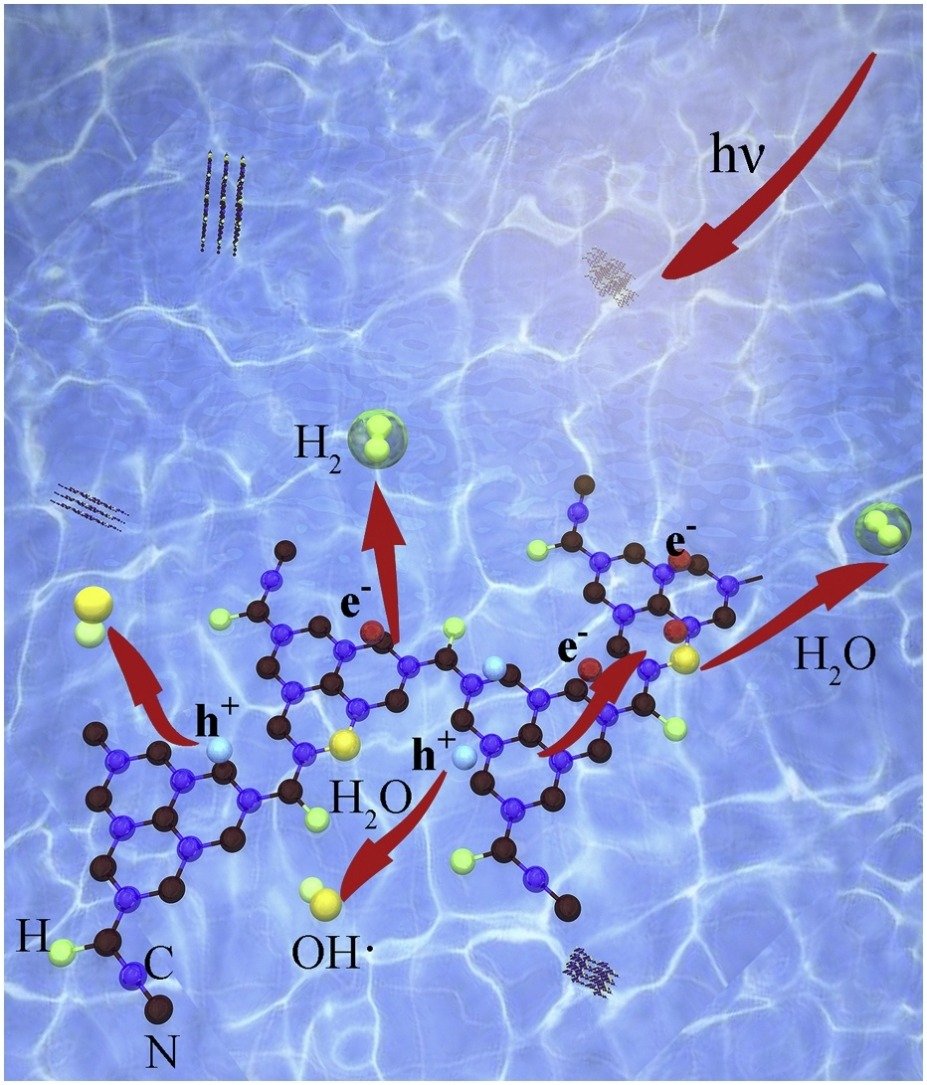

3. Polyaniline-grafted hydrolysed polyethylene as a dual functional interlayer/separator for high-performance Li-S@C core-shell batteries
By: Tubtimkuna, Suchakree; Krittayavathananon, Atiweena; Chiochan, Poramane; et al.
Chemical Communications, 2019, 55, 14263-14266
DOI: https://pubs.rsc.org/en/content/articlelanding/2019/CC/C9CC07725A#!divAbstract
Abstract: A modified hydrolysed polyethylene with polyaniline was used as a dual functional interlayer/separator for high-performance lithium–sulphur batteries (LSBs) to reduce the migration of soluble polysulphide intermediates. Also, the sulphur cathode was encapsulated with carbon nanoparticles with a S@C core–shell structure using a solvent-free coating process. The chemical interaction between the imine group of the quinoid ring in the PANI structure and the polysulphides reducing the shuttle effect as well as the high electrical conductivity and less volume change of the carbon encapsulated sulphur can provide high-performance Li–S@C core–shell batteries.

4. High-rate aqueous/ionic liquid dual electrolyte supercapacitor using 3D graphene sponge with an ultrahigh pore volume
By: Sathyamoorthi, Sethuraman; Chiochan, Poramane; Sawangphruk, Montree
Electrochimica Acta, 2019, 327, 135014
DOI: https://www.sciencedirect.com/science/article/pii/S0013468619318857?via%3Dihub
Abstract: Non-traditional electrolytes like “water-in-salt” and dual electrolyte are emerging as promising electrolytes for next-generation energy storage devices. However, their low rate capability is a major obstacle for their extensive employment. Here, we achieve a high-rate aqueous/ionic liquid dual electrolyte supercapacitor using 3D interconnected graphene sponge with a high pore volume of 5.6 cm3 g−1. It delivers a high rate of 30 A g−1 and the maximum cell potential of 2.0 V. The maximum specific energy of 9.1 Wh kg−1 at 0.5 A g−1 and the specific power of 16.3 kW kg−1 at 30 A g−1 are achieved for the dual electrolyte. Excellent long-term stability of 85% is estimated for the applied cell potential of 2.0 V after 50,000 cycles at 5 A g−1. In situ gas analysis using differential electrochemical mass spectrometry identifies three gases (H2, CO2 and CO) at the critical cell potentials. The possible cell potential-limiting electrode in the full cell is identified by the type of gas evolved at the maximum cell potentials. This high-rate supercapacitor may be useful for high-power applications.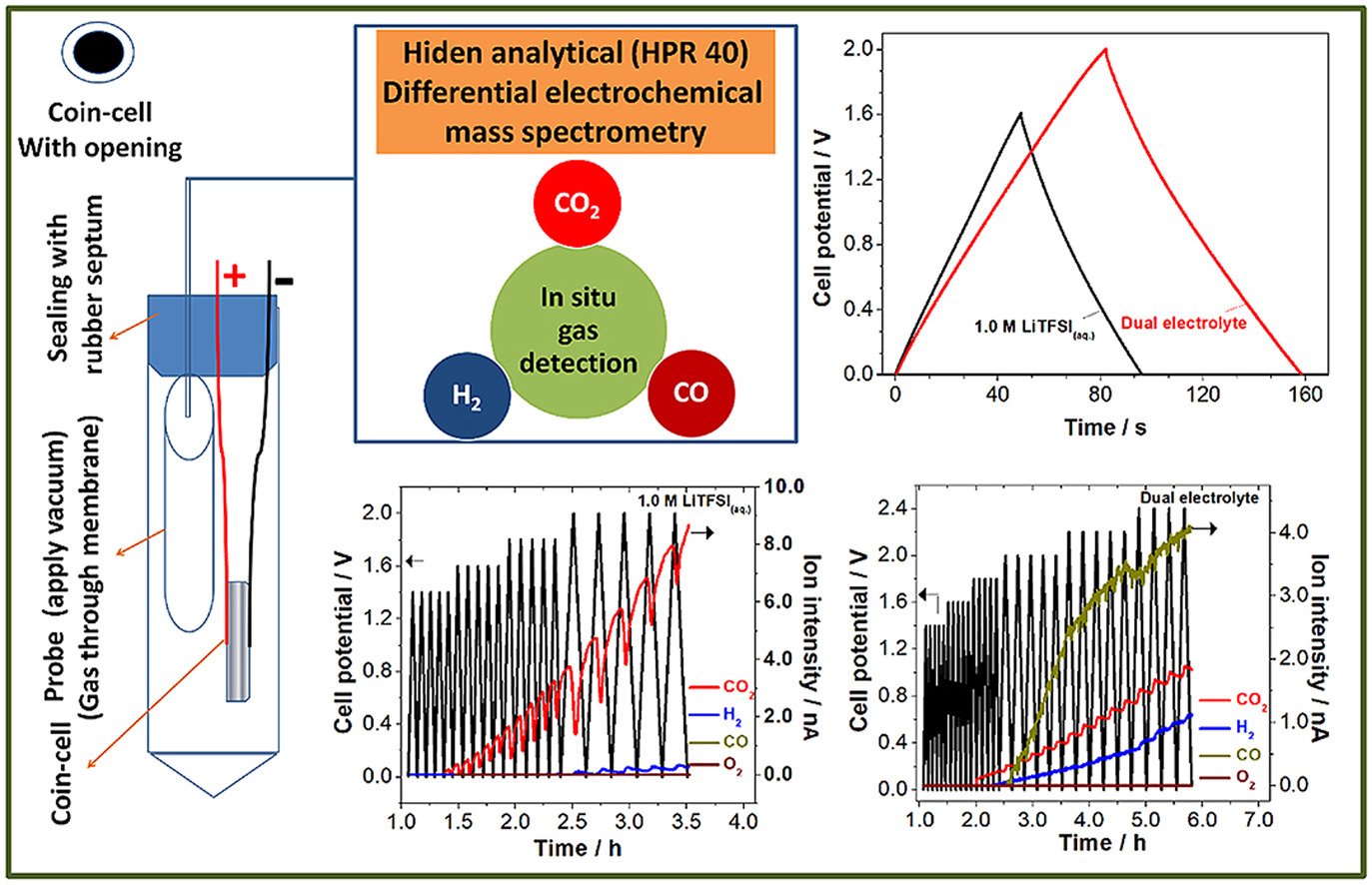

5. Insight into the effect of additives widely used in lithium-sulfur batteries
By: Duangdangchote, Salatan; Krittayavathananon, Atiweena; Phattharasupakun, Nutthaphon; et al.
Chemical Communications, 2019, 55, 13951-13954
DOI: https://pubs.rsc.org/en/content/articlelanding/2019/CC/C9CC06504K#!divAbstract
Abstract: The interaction between the reactive lithium metal surface and LiNO3 results in the formation of LixNOy clusters, which can protect the Li metal anode and suppress the shuttling effect of lithium polysulfides via the dipole–dipole interaction called the lithium bond.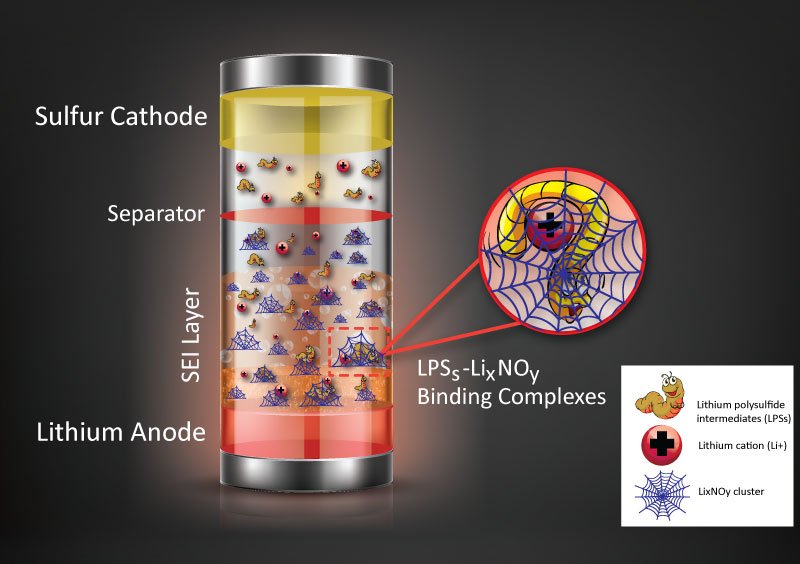

6. Strong cooperative interaction of lithium and hydrogen bonds between 4-aminobenzoic acid modified interlayer and polysulfides for lithium-sulfur batteries
By: Kosasang, Soracha; Ma, Nattapol; Duangdangchote, Salatan; et al.
CARBON, 2019, 155, 553-561
DOI: https://www.sciencedirect.com/science/article/pii/S0008622319309327?via%3Dihub
Abstract: Lithium-sulfur battery (LSB) is considered as a potential candidate for future energy storage device due to its high specific capacity and energy density as well as the natural abundance of sulfur. However, poor capacity retention, polysulfide shuttle effect, and low electronic conductivity have hindered practical uses of the LSB. The introduction of interlayer has been proven to be a promising strategy to improve the overall performance of the LSB. Herein, the influence of various amine-based functional groups of carbon interlayer on lithium polysulfide (LPS) chemisorption was compared via electrochemical methods and density functional theory (DFT) calculations. The functionalized carbon interlayers with 4-aminobenzoic acid, 1,6-diaminohexane, p-phenylenediamine, 4-nitroaniline, and 4-aminothiophenol prepared by an amide coupling reaction show a strong contribution to reduce the polysulfide migration, resulting in the enhancement of overall LSB performances such as a superior capacity and high Coulombic efficiency along with long cyclability of the cells. The LSB with 4-aminobenzoic acid could achieve an initial specific capacity of 1694 mAh g−1 (0.1 C) with an extremely low capacity decay of 0.055% per cycle due to the cooperative interaction of H- and Li-bonds between 4-aminobenzoic acid-functionalized interlayer and polysulfides.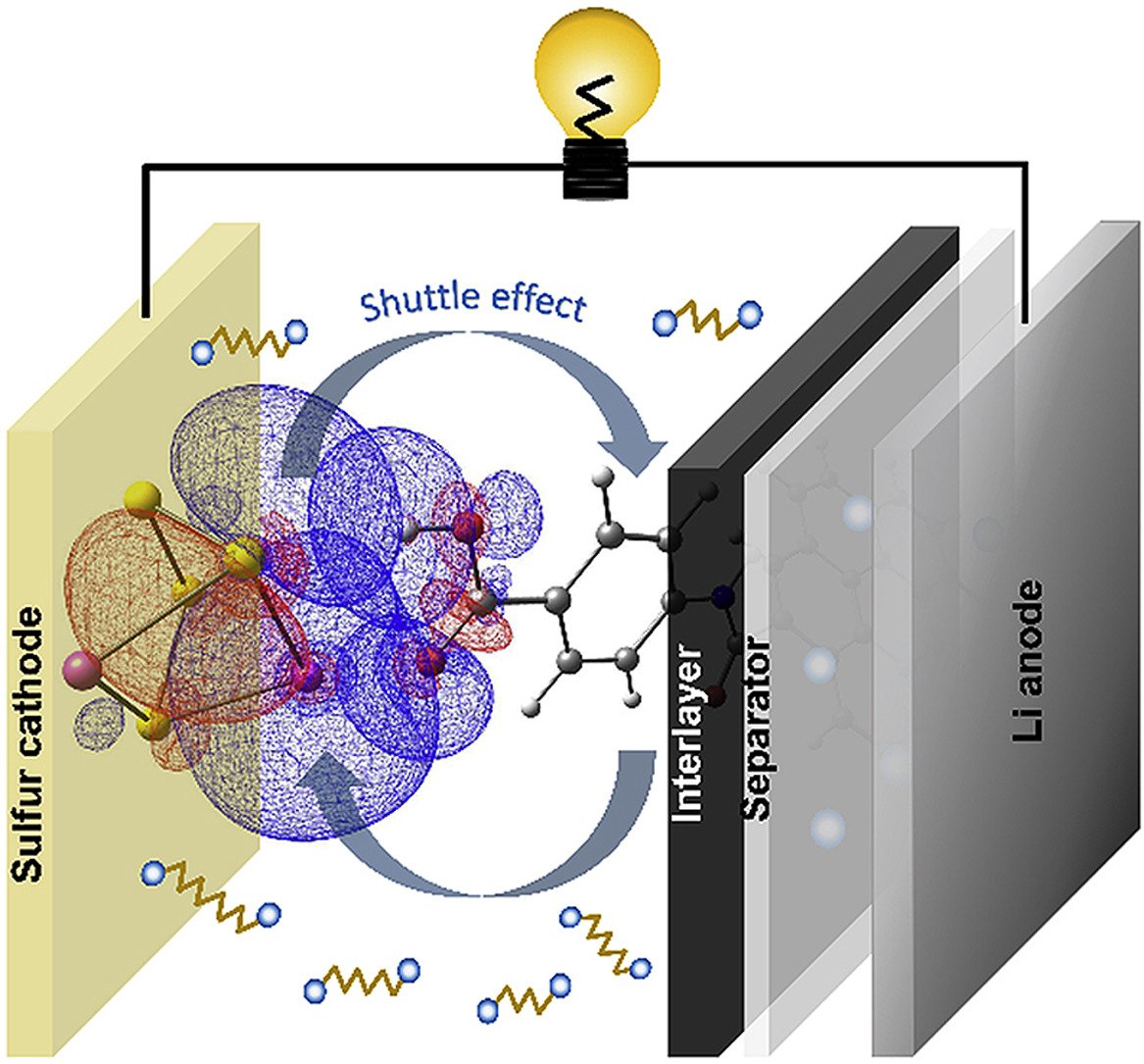

7. Charge storage mechanisms of cobalt hydroxide thin film in ionic liquid and KOH electrolytes for asymmetric supercapacitors with graphene aerogel
By: Kongsawatvoragul, Ketsuda; Kalasina, Saran; Kidkhunthod, Pinit; et al.
Electrochimica Acta, 2019, 324, 134854
DOI: https://www.sciencedirect.com/science/article/pii/S0013468619317256?via%3Dihub
Abstract: Layered 2D metal hydroxides and oxides with solvated ions inside their layers are well-known materials for energy storage applications. Although thin-film layered alpha-cobalt hydroxide (α-Co(OH)2) is recognized as one of the promising candidates among many other 2D materials, it still suffers from its poor stability in alkali solution i.e., 6 M KOH. In this work, the charge storage mechanism and the electrochemical performance of α-Co(OH)2 in a room-temperature ionic liquid (IL) namely 1-butyl-1-methyl-pyrrolidinium dicyanamide ionic liquid ([BMPyr+][DCA−] IL) were investigated and compared with those in 6 M KOH. Interestingly, α-Co(OH)2 in [BMPyr+][DCA−] IL maintains their capacity retention up to 95.9% after 10,000 cycles at 0.15 mA cm−2 for the half-cell evaluation. The asymmetric cell of thin-film α-Co(OH)2//reduced graphene oxide aerogel (rGOae) assembled in [BMPyr+][DCA−] IL electrolyte provides a high areal capacitance of 18.54 F cm−2 (28.6 F g−1) at 0.15 mA cm−2 as compared with other thin-film supercapacitors. The charge storage mechanism of α-Co(OH)2 in [BMPyr+][DCA−] IL investigated by in situ X-ray absorption spectroscopy (XAS) and ex situ X-ray photoelectron spectroscopy (XPS) confirms that [DCA−] can faradaically react with α-Co(OH)2 providing CoOOH and NH(CN)2. Understanding charge storage mechanism of 2D metal hydroxides may be useful for future development of energy storage technology.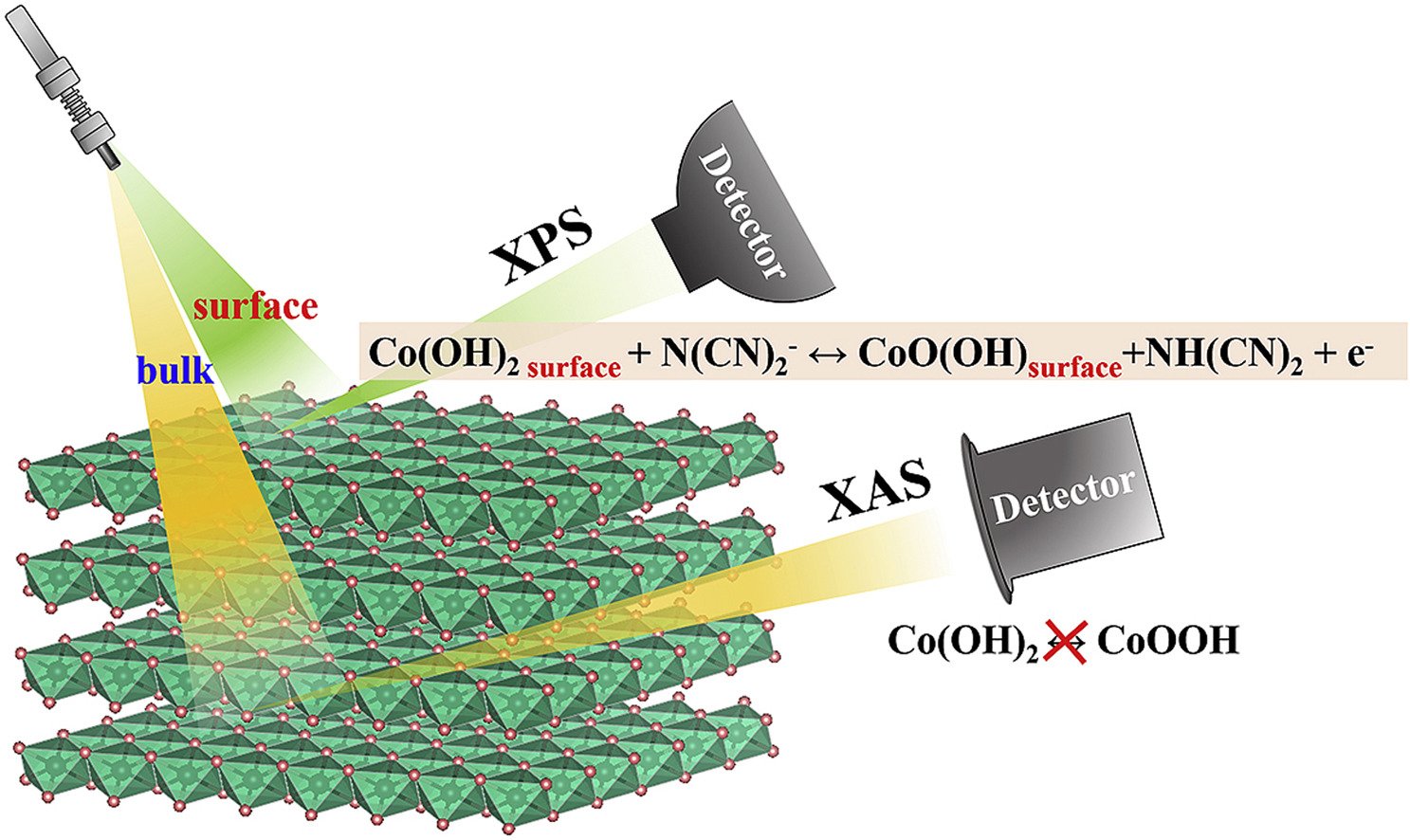

8. 3D CVD graphene oxide-coated Ni foam as carbo- and electro-catalyst towards hydrogen evolution reaction in acidic solution: In situ electrochemical gas chromatography
By: Sarawutanukul, Sangchai; Phattharasupakun, Nutthaphon; Sawangphruk, Montree
CARBON, 2019, 151, 109-119
DOI: https://www.sciencedirect.com/science/article/pii/S0008622319305317?via%3Dihub
Abstract: 3D graphene oxide (GO) carbocatalyst-coated nickel foam substrate (GO@Ni) was synthesized by chemical vapor deposition (CVD) using methanol and water as material precursors. Interestingly, the GO@Ni has anti-corrosion property in an acidic electrolyte and high catalytic activity towards hydrogen evolution reaction (HER). Ni metal is easily corroded in the acid solution and cannot be used as an electrocatalyst in electrolysers for hydrogen generation. On the other hand, the GO@Ni with oxygen-containing functional groups exhibits a high HER catalytic activity with an overpotential of 83.2 mV at a current density of 10 mA cm−2. It can also be continuously tested under 1.0 M H2SO4 for 30 h without a corrosion issue of Ni substrate. In addition, the change in the morphological and structural properties of the GO@Ni after testing in H2SO4 was investigated by microscopy and spectroscopy techniques. The rate of hydrogen evolution was found to be in the order of 10−7 mol cm−2 s−1 as measured by an in situ electrochemical gas chromatography (GC). The GO@Ni electrode could be an alternative electrocatalyst in the electrolysers towards HER under acidic environment.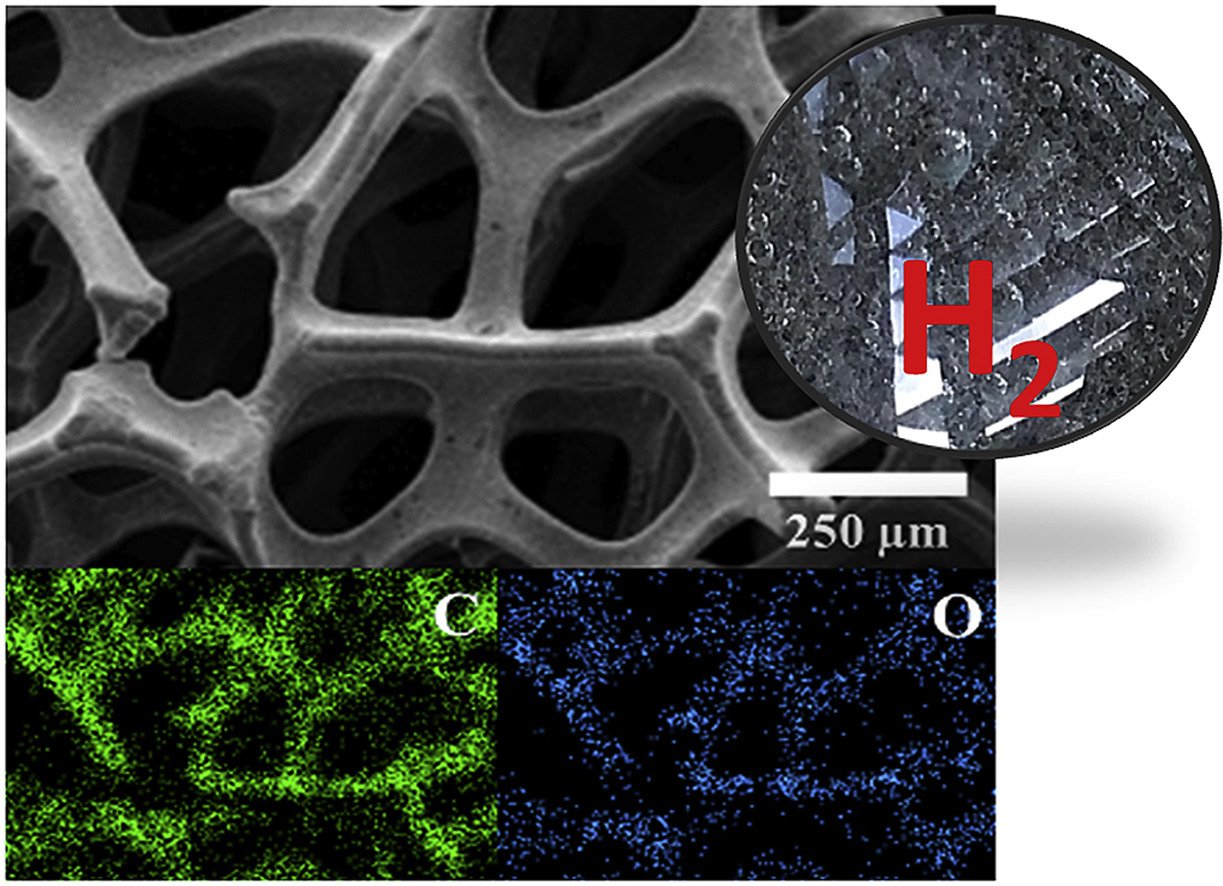

9. In situ mass change and gas analysis of 3D manganese oxide/graphene aerogel for supercapacitors
By: Suktha, Phansiri; Chiochan, Poramane; Krittayavathananon, Atiweena; et al.
RSC Advances, 2019, 9, 28569-28575
DOI: https://pubs.rsc.org/en/content/articlelanding/2019/ra/c9ra05444h#!
Abstract: Manganese oxide nanoparticles decorated on 3D reduced graphene oxide aerogels (3D MnOx/rGOae) for neutral electrochemical capacitors were successfully produced by a rapid microwave reduction process within 20 s. The symmetric electrochemical capacitor of 3D MnOx/rGOae (Mn 3.0 at%) storing charges via both electric double layer capacitance (EDLC) and pseudocapacitance mechanisms exhibits a specific capacitance of 240 F g−1 as compared with 190 F g−1 of that using the bare 3D rGOae at 0.5 A g−1 in 1 M Na2SO4 (aq.) electrolyte. It retains 90% of the initial capacitance after 10 000 cycles, demonstrating high cycling stability. In addition, the charge storage mechanism of 3D MnOx/rGOae was investigated using an electrochemical quartz crystal microbalance. In situ gas analysis using differential electrochemical mass spectrometry (DEMS) shows the CO2 evolution at a cell potential over 1 V indicating that the positive electrode is possibly the voltage limiting electrode in the full cell. This finding may be useful for further development of practical high power and energy supercapacitors.
10. High-Performance Li-Ion Batteries Using Nickel-Rich Lithium Nickel Cobalt Aluminium Oxide-Nanocarbon Core-Shell Cathode: In Operando X-ray Diffraction
By: Vadivel, Selvamani; Phattharasupakun, Nutthaphon; Wutthiprom, Juthaporn; et al.
ACS APPLIED MATERIALS & INTERFACES, 2019, 11, 30719-30727
DOI: https://pubs.acs.org/doi/10.1021/acsami.9b06553
Abstract: Nickel-rich layered, mixed lithium transition-metal oxides have been pursued as a propitious cathode material for the future-generation lithium-ion batteries due to their high energy density and low cost. Nevertheless, acute side reactions between Ni4+ and carbonate electrolyte lead to poor cycling as well as rate performance, which limits their large-scale applications. Here, core–shell like LiNi0.8Co0.15Al0.05O2 (NCA)–carbon composite synthesized by a solvent-free mechanofusion method is reported to solve this issue. Such a core–shell structure exhibits a splendid rate as well as stable cycling when compared to the physically blended NCA. In operando X-ray diffraction studies show that both materials experience anisotropic structural change, i.e., stacking c-axis undergoes a gradual expansion followed by an abrupt shrinkage; meanwhile, the a-axis contracts during the charging process and vice versa. Interestingly, the core–shell material displays a significantly high reversible capacity of 91% in the formation cycle at 0.1C and a retention of 84% at 0.5C after 250 cycles, whereas pristine NCA retains 71%. The robust mechanical force assisted dry coating obtained by the mechanofusion method shows improved electrochemical performance and demonstrates its practical feasibility.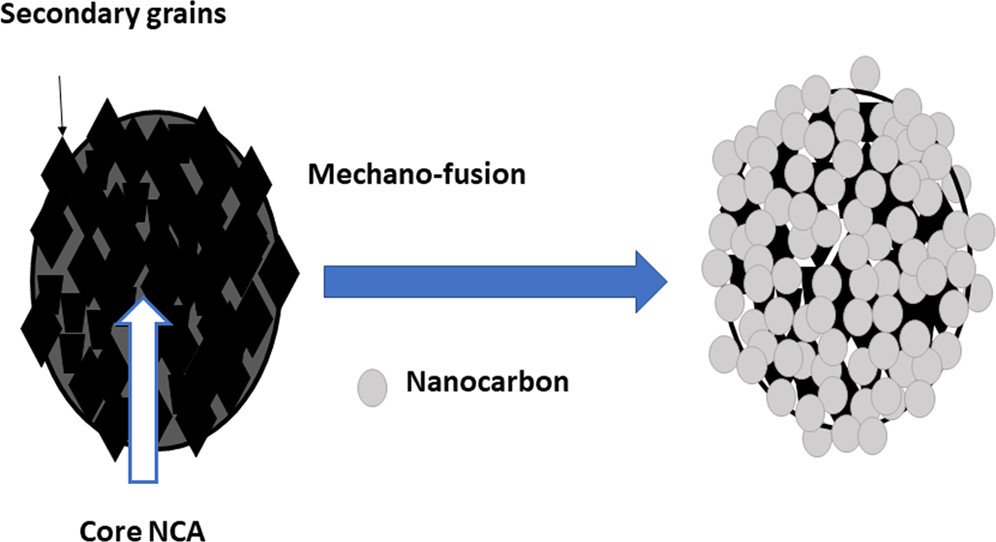

11. High cell-potential and high-rate neutral aqueous supercapacitors using activated biocarbon: In situ electrochemical gas chromatography
By: Le Calvez, Etienne; Sathyamoorthi, Sethuraman; Phattharasupakun, Nutthaphon; et al.
Electrochimica Acta, 2019, 313, 31-40
DOI: https://www.sciencedirect.com/science/article/pii/S0013468619309454?via%3Dihub
Abstract: Electric double layer capacitors (EDLCs) are known for their high-power densities due to the fast mechanism of charge-discharge processes. However, producing eco-friendly and economically viable materials for supercapacitors is one of the most important challenges. Also, a high-rate capability of the supercapacitor is desirable for pulse applications. In the present work, a high cell-potential and high-rate supercapacitor was created using biocarbon derived from a rubberwood-sawdust biomass at three different temperature. Bio-AC carbonized at 900 °C shows a high specific surface area of 1932 m2 g−1 and a pore volume of 1.44 cm3 g−1. A symmetrical supercapacitor of bio-AC(900 °C) with 1.0 M LiTFSI(aq.) displays a high practical cell potential of 1.8 V with an impressive rate up to 100 A g−1. A specific capacitance of ∼100 F g−1 at 0.1 A g−1 was estimated by a galvanostatic charge-discharge method. Besides, an excellent capacitance retention is retained at higher specific currents, for instance, 71% and 49% were estimated at 60 A g−1 and 100 A g−1, respectively. An excellent long-term stability of 84% at 1.8 V was evaluated for the symmetrical supercapacitor in 1.0 M LiTFSI(aq.) after 50,000 cycles at 3.0 A g−1. Also, gas chromatography coupled with the electrochemical cell was employed for the in situ determination of electrolytically generated gases in real time at the positive and negative applied potentials. H2 and CO2 gasses can be generated at too high applied potentials. Understanding the gas evolution in this work may be useful for further development of supercapacitors. Also, high cell-potential and high-rate neutral aqueous supercapacitors using activated biocarbon may be useful for high-power applications.
12. SiCx/TiCx Nanostructured Material from Ti3SiC2 for High Rate Performance of Lithium Storage
By: Li, Chenyang; Xue, Zhe; Qin, Jiaqian; et al.
CHEMISTRYSELECT, 2019, 4, 7766-7772
DOI: https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/slct.201901318
Abstract: A new SiCx/TiCx electrode material has been synthesized by ball‐milling of Ti3SiC2 MAX phase. To demonstrate the possible applications for lithium‐ion batteries, the electrochemical properties of the SiCx/TiCx nanocomposite was studied. The results indicate that this new material has a higher specific capacity (170 mAh/g) than those of pure SiC (93 mAh/g), TiC (75 mAh/g), and the mixture of SiC‐TiC (122 mAh/g) at current density of 100 mA/g for 300 cycles. Furthermore, the SiCx/TiCx nanocomposite has a specific capacity of 110 mAh/g, better than those of artificial graphite (labeled: SAG−R, 26 mAh/g) and mesocarbon microbeads (labeled: MCMB, 21 mAh/g), at current density of 1 A/g for 1000 cycles. The enhancement mechanism of the SiCx/TiCx nanostructure has been discussed by the DFT calculation. Further development of this anode material may enable practical applications for long cycling and fast charge‐discharge anode in rechargeable batteries.
13. Thin-Film Photoelectrode of p-Type Ni-Doped Co3O4 Nanosheets for a Single Hybrid Energy Conversion and Storage Cell
By: Kalasina, Saran; Kongsawatvoragul, Ketsuda; Phattharasupakun, Nutthaphon; et al.
Journal of The Electrochemical Society, 2019, 166, A2444-A2452
DOI: https://iopscience.iop.org/article/10.1149/2.0351912jes
Abstract: A thin-film Ni0.07Co2.93O4 p-type semiconductor finely tuned with better electronic conductivity and electrochemical activity than NiO and Co3O4 is proposed as a new photoelectrode of single asymmetric hybrid energy conversion and storage cell (HECS). Photoactive Ni0.07Co2.93O4 and N-doped reduced graphene oxide aerogel (N-rGOAE) were used as positive and negative electrodes of the HECS. Under light illumination, the Ni0.07Co2.93O4 can generate a hole (photogenerated carrier, Ni0.07Co2.93O4+) at the valence band (VB) and a photoelectron at the conduction band (CB) via the photoelectric effect. During charging, the active hole (Ni0.07Co2.93O4+) of the positive electrode can chemically react with OH− and H2O forming CoO(OH) and NiO(OH). This process can enhance the redox mechanism leading to the charge storage performance of the Ni0.07Co2.93O4 electrode since OH− can easily react with the active Ni0.07Co2.93O4+ hole having the lower energy level of the VB when compared with the electrode under the dark condition. The changes in the oxidation state of Ni and Co in Ni0.07Co2.93O4 were fundamentally investigated by an in situ electrochemical X-ray absorption spectroscopy. The electrochemical performance of Ni0.07Co2.93O4//N-rGOAE HECS can be enhanced under light illumination about 1.9-fold higher than that under dark condition. The HECS device may be useful for renewable energy applications.
14. High-performance spinel LiMn2O4@carbon core-shell cathode materials for Li-ion batteries
By: Selvamani, Vadivel; Phattharasupakun, Nutthaphon; Wutthiprom, Juthaporn; et al.
Sustainable Energy & Fuels, 2019, 3, 1988-1994
DOI: https://pubs.rsc.org/en/content/articlelanding/2019/SE/C9SE00274J#!divAbstract
Abstract: A core–shell-type spinel LiMn2O4/carbon composite was synthesized by a simple and cost-effective mechanofusion method (dry particle coating) with a highly uniform coating. Electrochemical characterizations demonstrated that the surface-engineered core–shell-like material exhibited superior rate retention as well as cycling stability than pristine LMO due to improved intrinsic conductivity and easy electrolyte access. As a result, in the half-cell configuration, the core–shell carbon composite delivered reversible specific capacity of 103 mA h g−1 after 1000 cycles at 0.75C with 82% capacity retention; however, the pristine material showed specific capacity of 78 mA h g−1 and 76% capacity retention after 600 cycles. Similarly, in the full cell studies, the core–shell material exhibited 70% capacity retention, whereas the pristine material retained only 53% after 1000 cycles at 0.1 A g−1. The spinel LiMn2O4@carbon core–shell material obtained by the mechanofusion method may be a practical cathode material in high-performance lithium-ion batteries toward high energy applications.
15. Photoactive Zn-air batteries using spinel-type cobalt oxide as a bifunctional photocatalyst at the air cathode
By: Tomon, Chanikarn; Sarawutanukul, Sangchai; Duangdangchote, Salatan; et al.
Chemical Communications, 2019, 55, 5855-5858
DOI: https://pubs.rsc.org/en/content/articlelanding/2019/CC/C9CC01876J#!divAbstract
Abstract: Spinel-type cobalt oxide (Co3O4) was synthesized and used as a photoactive bifunctional electrocatalyst towards the oxygen evolution reaction (OER) and the oxygen reduction reaction (ORR) at the air cathode of zinc–air batteries (ZABs). The Co3O4 having direct and indirect band gap energies of ca. 2.20 eV and ca. 1.35 eV can absorb visible light, generating photogenerated carriers and photoelectrons via the photoelectric effect. Upon exposure to visible light, the Co3O4 electrode exhibits ca. 30% higher current density than that under dark conditions and provides around 10–20% lower OER and ORR overpotentials than those under the dark conditions. Under visible light, the specific capacity of the as-fabricated photoactive ZAB cell is improved by ca. 10% as compared to that under dark conditions.
16. A 3D free-standing lithiophilic silver nanowire aerogel for lithium metal batteries without lithium dendrites and volume expansion: in operando X-ray diffraction
By: Phattharasupakun, Nutthaphon; Wutthiprom, Juthaporn; Duangdangchote, Salatan; et al.
Chemical Communications, 2019, 55, 5689-5692
DOI: https://pubs.rsc.org/en/content/articlelanding/2019/CC/C9CC01528K#!divAbstract
Abstract: A 3D free-standing lithiophilic silver nanowire aerogel (AgNWA) can stop the dendritic growth of lithium metal at the initial nucleation process. The 3D structure can also suppress the infinite volume expansion of lithium during cycling. The active AgNWA scaffold can serve as a Li reservoir to compensate for the irreversible consumption of Li. The lithiated AgNWA anode was coupled with a lithium iron phosphate (LFP) cathode in a full-cell configuration providing much higher performance than the Li//LFP cell.
17. A simple and practical hybrid ionic liquid/aqueous dual electrolyte configuration for safe and ion-exchange membrane-free high cell potential supercapacitor
By: Sathyamoorthi, Sethuraman; Sawangphruk, Montree
Electrochimica Acta, 2019, 305, 443-451
DOI: https://www.sciencedirect.com/science/article/pii/S0013468619304979?via%3Dihub
Abstract: A simple and practical hybrid dual ionic liquid (IL)/1.0 M LiTFSI(aq.) is proposed using ILs in the nanopores of activated carbon for high cell potential supercapacitor. Unlike previously reported system, the proposed supercapacitors use no ion-exchange membrane, a low quantity of ILs (∼20 μL/cell) and a simple coin cell (single compartment). Hydrophilic and hydrophobic ILs were tested in the hybrid dual electrolyte configuration. The dual electrolyte with hydrophobic ILs enables the maximum operational cell potential of 2.2 V with the excellent capacitance retention of 94% after continuous charge-discharge cycles up to 30,000 at 0.5 A g−1. The identified maximum cell potential is higher than the previously reported value of 1.9 V for the supercapacitors with the dual electrolyte comprising an ion-exchange membrane and, also comparable with the supercapacitors using “water-in-salt” electrolytes (2.2 V). The supercapacitor cell using proposed hybrid dual IL/aqueous electrolyte delivers the specific capacitance of 34.0 F g−1 and the specific energy of 22.0 Wh kg−1. The proposed hybrid dual IL/aqueous electrolyte may be useful for many energy storage devices requiring high cell potentials.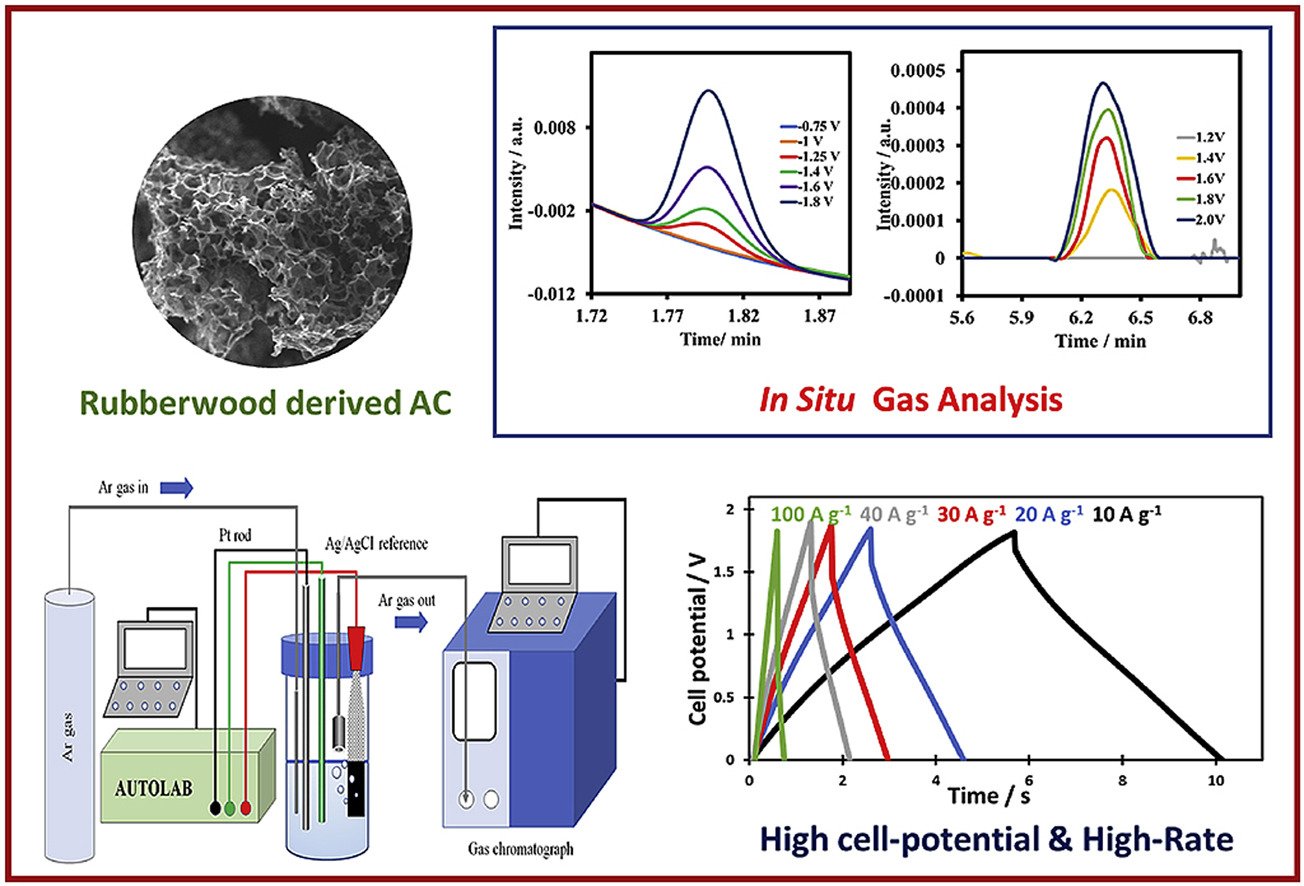

18. Lithium Intercalated-Layered Manganese Oxide and Reduced Graphene Oxide Composite as a Bifunctional Electrocatalyst for ORR and OER
By: Kosasang, Soracha; Ma, Nattapol; Phattharasupakun, Nutthaphon; et al.
Journal of The Electrochemical Society, 2019, 166, A1543-A1549
DOI: https://iopscience.iop.org/article/10.1149/2.0901908jes
Abstract: The bifunctional electrocatalytic activities of birnessite-type layered manganese oxide with lithium ion as an intercalated cation (Li-bir) and its composite with reduced graphene oxide (Li-bir/rGO) toward oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) were investigated. The enhanced catalytic activity of the Li-bir/rGO composite can be ascribed to the elevated electrical conductivity of the birnessite suggested via a lower charge transfer resistance obtained from the electrochemical impedance spectroscopy. Moreover, the local oxidation states of Mn in the Li-bir/rGO were investigated by in situ X-ray absorption spectroscopy during the ORR and OER processes. Interestingly, the generated oxygen gas from the OER process observed via the in situ gas chromatography of the Li-bir/rGO is 5.44 mmol g−1 which is higher than that of the pristine Li-bir (4.98 mmol g−1). These results suggest that the Li-bir/rGO can be further utilized as a bifunctional electrocatalyst for high-performance metal-air batteries.
19. Addition of Redox Additive to Ionic Liquid Electrolyte for High-Performance Electrochemical Capacitors of N-Doped Graphene Aerogel
By: Ma, Nattapol; Kosasang, Soracha; Phattharasupakun, Nutthaphon; et al.
Journal of The Electrochemical Society, 2019, 166, A695-A703
DOI: https://iopscience.iop.org/article/10.1149/2.0831904jes
Abstract: To enhance the specific energy of electrochemical capacitors, several methods have been introduced including complex electrode modification as well as asymmetric cell development. Herein, an alternative approach to enhance both specific energy and power of N-doped reduced graphene oxide aerogel electrochemical capacitor via the introduction of hybrid redox electrolyte is proposed. The electrochemical properties of the hybrid electrolyte composing of 1-butyl-1-methylpyrrolidinium dicyanamide ionic liquid with 100 mM ferrocene methanol redox additive were studied via cyclic voltammetry, galvanostatic charge-discharge, and electrochemical impedance spectroscopy. The combination between a unique nanostructure of N-doped reduced graphene oxide aerogel and novel hybrid electrolyte results in an excellent specific capacitance and specific energy of 112.1 F g−1 and 34.2 Wh kg−1, respectively, as compared to 76.7 F g−1 and 23.5 Wh kg−1 of the neat 1-butyl-1-methylpyrrolidinium dicyanamide electrolyte. The remarkable improvements can be explained by the emerging of the Faradaic-redox activity of the ferrocene methanol at the electrode-electrolyte interface. This simple approach could demonstrate another feasible route to improve the performance of ionic liquid-based electrochemical capacitors.
20. Visible Light-Driven Photocatalytic H-2 Generation and Mechanism Insights into Bi2O2CO3/G-C3N4 Z-Scheme Photocatalyst
By: Yang, Chengwu; Xue, Zhe; Qin, Jiaqian; et al.
Journal of Physical Chemistry, 2019, 123, 4795–4804
DOI: https://pubs.acs.org/doi/10.1021/acs.jpcc.8b10604
Abstract: Developing a low-cost photocatalyst with efficient performance is significant for practical application of solar-to-fuel conversion. Here, we first adopt a facile method to synthesize Bi2O2CO3-modified g-C3N4 heterojunction via in situ thermal growth. Bi2O2CO3 nanoparticles on g-C3N4 nanosheets play a vital role in improving the photocatalytic activity of splitting water for hydrogen production. The activity of Bi2O2CO3/g-C3N4 heterojunction during 5 h reaches 965 μmol·g–1·h–1, which is much higher than that of pure g-C3N4 (337 μmol·g–1·h–1) or other modified g-C3N4 materials. The significantly enhanced photocatalytic activity is attributed to direct Z-scheme system construction, resulting in a superior charge carrier separation ability. Theoretical calculations further reveal the redistribution of charge carrier at interface between Bi2O2CO3 and g-C3N4. This work provides new direction to synthesize g-C3N4-based heterojunction with high photocatalytic performance for alleviating energy and environmental issues.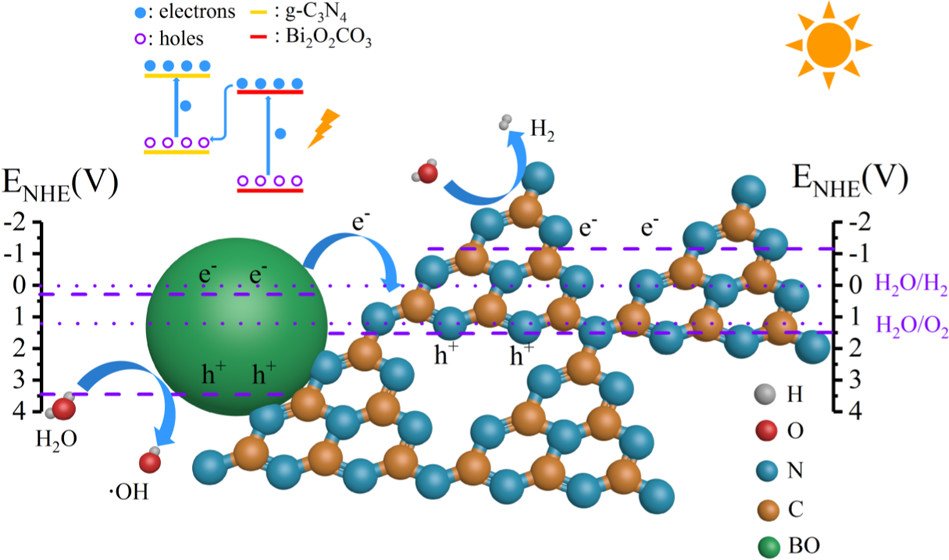

21. Porous Fe-N-C Catalysts for Rechargeable Zinc-Air Batteries from an Iron-Imidazolate Coordination Polymer
By: Lee, Jet-Sing M.; Sarawutanukul, Sangchai; Sawangphruk, Montree; et al.
ACS Sustainable Chemistry & Engineering, 2019, 7, 4030–4036
DOI: https://pubs.acs.org/doi/10.1021/acssuschemeng.8b05403
Abstract: Zn–air batteries are superior energy storage devices to Li-ion, possessing larger storage capacities with the benefits of high safety and economic viability. The large-scale use of this technology requires development of cheap and efficient bifunctional electrocatalysts for the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) processes. Herein, a highly active bifunctional porous Fe–N–C catalyst was synthesized from a low-cost iron-imidazolate framework template. The optimized catalyst exhibits high performances for both ORR and OER processes in alkali media, with oxygen electrode activities which outperform benchmark platinum-based catalysts. Rechargeable Zn–air batteries were constructed and exhibited low overpotentials, and high stability, showing the prospect of these nonprecious metal bifunctional catalysts in metal–air batteries.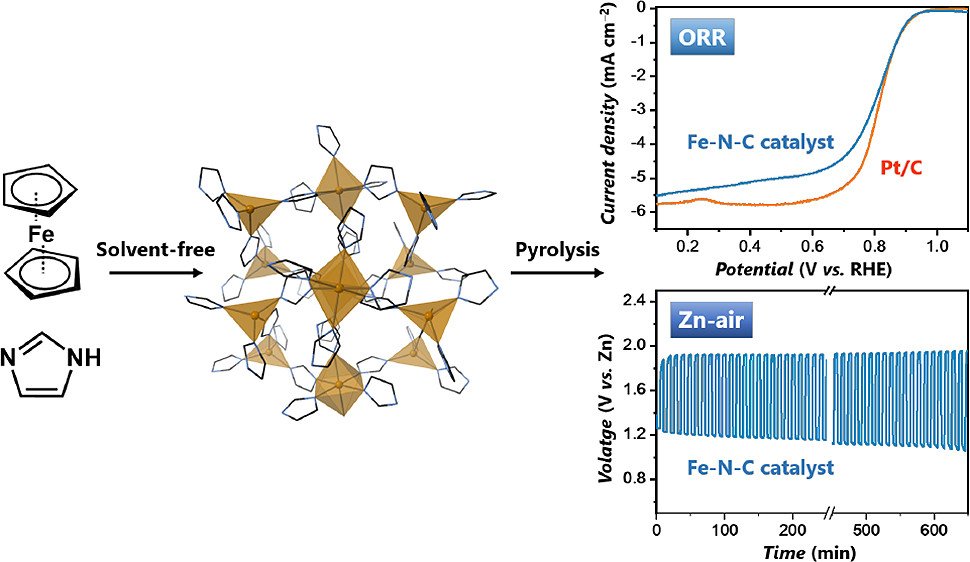

22. Lightweight Multi-Walled Carbon Nanotube/N-Doped Graphene Aerogel Composite for High-Performance Lithium-Ion Capacitors
By: Aphirakaramwong, Chalita; Phattharasupakun, Nutthaphon; Suktha, Phansiri; et al.
Journal of The Electrochemical Society, 2019, 166, A532-A538
Abstract: Graphene composite with carbon nanotube is one of promising active materials for energy storage applications. Herein, the N-doped reduced graphene oxide/reduced multi-walled carbon nanotube oxide (N-rGOae/r-MWCNTO) composite was inspirationally introduced as the negative electrode material for the lithium hybrid capacitors (LICs). The negative electrodes of the N-rGOae/r-MWCNTO composites with respective to Li/Li+ initially deliver ca. 1500–2900 mAh g−1 at 0.1 A g−1 for which the electrode consisting of 50:50 wt% of N-rGOae: r-MWCNTO finely tuned provides the maximum specific capacity with a cycling stability over 500 cycles with 100% coulombic efficiency. In addition, the full cell of 50:50 wt% N-rGOae/r-MWCNTO//activated carbon (negative electrode//positive electrode) provides a voltage window of 2.0–4.0 V, an impressively maximum energy density of 189.90 Wh kg−1 and a power density of 24105 W kg−1 based on the active materials from both electrodes. As a result, this device may be useful for high energy and power applications.
23. Effect of intercalated alkali ions in layered manganese oxide nanosheets as neutral electrochemical capacitors
By: Ma, Nattapol; Kosasang, Soracha; Krittayavathananon, Atiweena; et al.
Chemical Communications, 2019, 55, 1213-1216
DOI: https://pubs.rsc.org/en/content/articlelanding/2019/CC/C8CC08198K#!divAbstract
Abstract: New insight into the influence of Li+, Na+, and K+ cations between adjacent layers of birnessite-type manganese oxides (MnOx) towards the intercalation/deintercalation charge storage mechanism as a neutral electrochemical capacitor (1 M Na2SO4) is demonstrated. These structural cations play a major role in both the kinetic electron transfer in a faradaic redox reaction and the accessibility of the compensating electrolyte ions. Li-MnOx shows the highest Mn utilization of 51% followed by Na-MnOx (40%) and K-MnOx (31%), respectively.