1. Insight into the charge storage mechanism and capacity retention fading of MnCo2O4 used as supercapacitor electrodes
By: Krittayavathananon, Atiweena; Pettong, Tanut; Kidkhunthod, Pinit; et al.
Electrochimica Acta, 2017, 258, 1008-1015
DOI : https://www.sciencedirect.com/science/article/pii/S0013468617325148?via%3Dihub
Abstract: Although an inverse-spinel MnCo2O4 has been widely used as a promising supercapacitor material due to its high theoretical capacitance (>3000 F g−1), the supercapacitor device of MnCo2O4 exhibits poor capacity retention, only 79% after 2500 cycles, limiting its practical application. In situ electrochemical X-ray absorption spectroscopy (XAS), in situ mass detection electrochemical quartz crystal microbalance (EQCM), ex situ field emission scanning electron microscopy (FESEM), and ex situ extended X-ray absorption fine structure (EXAFS) were used to investigate charge storage mechanism of the MnCo2O4 and the origin of its capacity retention fading. It was found that the capacity retention of MnCo2O4 supercapacitors is sensitive to the concentration of electrolyte. High concentration (e.g., 6 M) of KOH electrolyte provides poor capacity retention due to the Jahn-Teller distortion at the positive electrode and the phase transformation at the negative electrode. More interestingly, the MnCo2O4 used at the negative electrode is less stable than that used at the positive electrode. Using low concentration of KOH (i.e., 1 M) and the asymmetric supercapacitor design using MnCo2O4 at the positive electrode would enhance their charge storage performance with high capacity retention.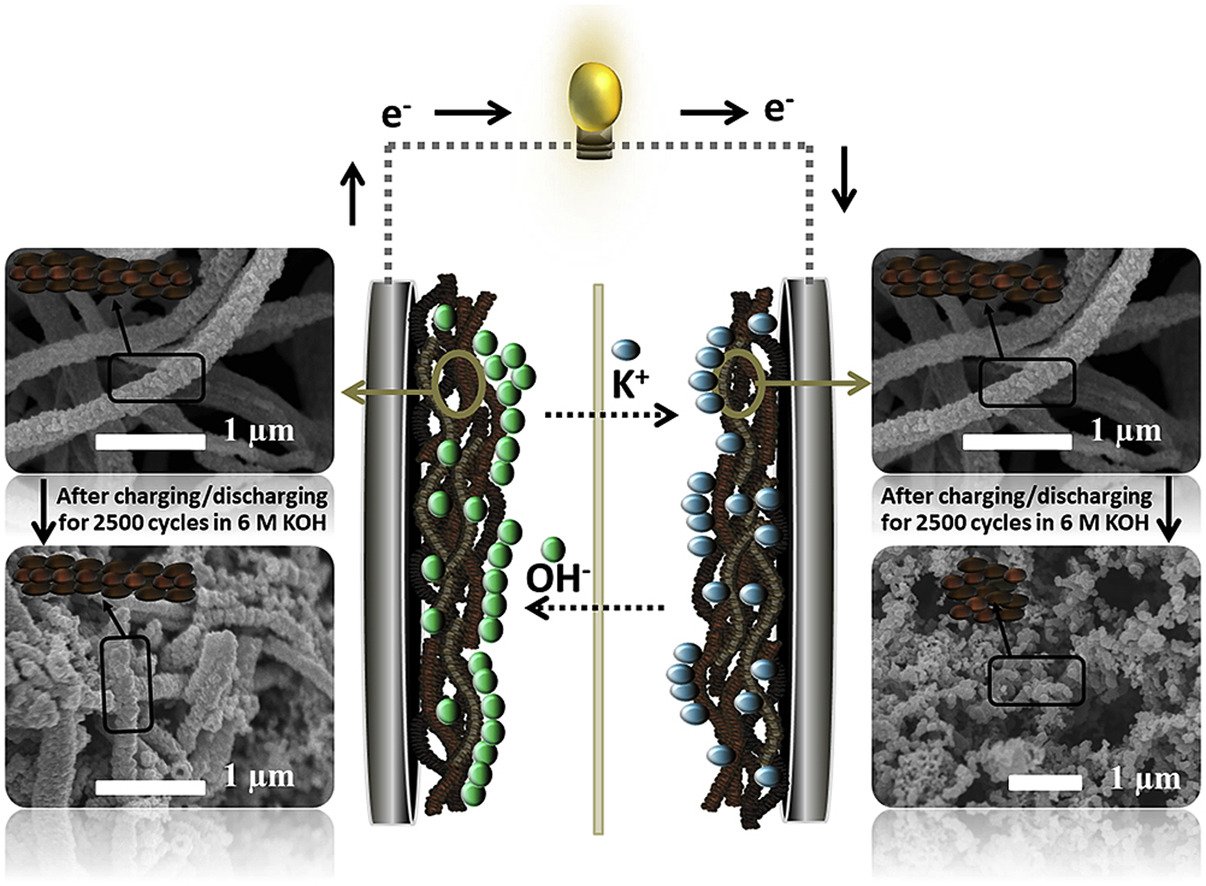

2. Impedimetric Sensor of ss-HSDNA/Reduced Graphene Oxide Aerogel Electrode toward Aflatoxin B1 Detection: Effects of Redox Mediator Charges and Hydrodynamic Diffusion
By: Krittayavathananon, Atiweena; Sawangphruk, Montree
ANALYTICAL CHEMISTRY, 2017, 89, 13283–13289
DOI : https://pubs.acs.org/doi/10.1021/acs.analchem.7b03329
Abstract: Here, an impedimetric biosensor for determination and quantification of an aflatoxin B1 (AFB1) level using a reduced graphene oxide aerogel labeled with a single strand DNA (ss-HSDNA/rGOae) modified on a rotating disk electrode (RDE) is presented. Owing to the large biomolecule biding on the electrode, an electron transfer is interrupted and not easily accessible to a target molecule. To address this issue, we aim to study two effects; one considers electro-redox mediators and the other considers the hydrodynamic effect. By observing a cyclic voltammetric response from the ss-HSDNA/rGOae electrode in three different charges of the redox mediators (i.e., neutral FcCH2OH, cationic Ru(NH3)63+, and anionic Fe(CN)64–) in a phosphate buffer solution (PBS) containing AFB1, the magnitude of anodic current at 50 mV s–1 is 825, 615, and 550 mA cm–1, respectively, which is significant dominated by the charge of the redox probe. The effect of hydrodynamic diffusion of the ss-HSDNA/rGOae rotating disk electrode (RDE) toward AFB1 detection using FcCH2OH as the redox mediator was recorded by applying a range of rotating speed from 500 to 4000 rpm. Increasing rotating speed reduces the charge transfer resistance resulting in the lower detectable level for AFB1 quantification. In the case of 4000 rpm, the AFB1 can be detected with a limit of detection of 0.04 ng/mL and a linear range of 1 × 10–10 to 7 × 10–8g/mL.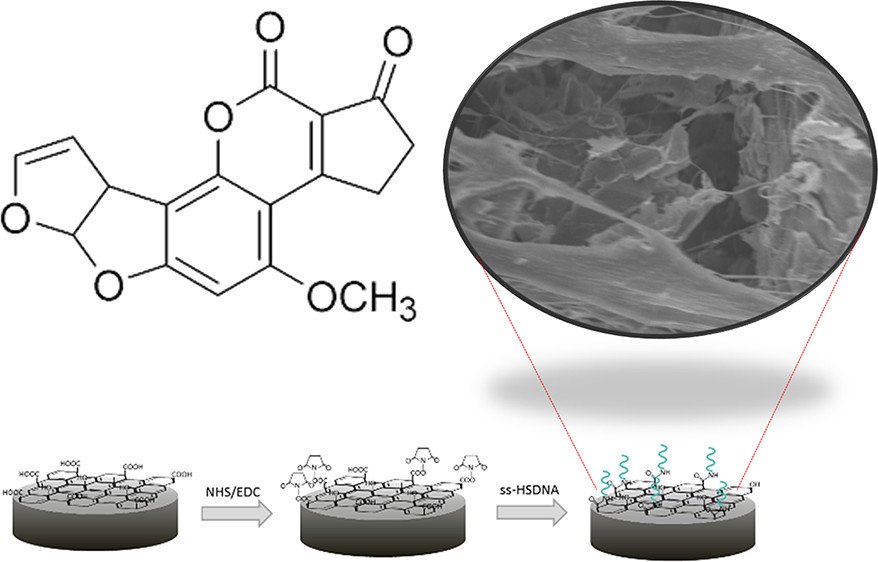

3. Chemical Adsorption and Physical Confinement of Polysulfides with the Janus-faced Interlayer for High-performance Lithium-Sulfur Batteries
By: Chiochan, Poramane; Kaewruang, Siriroong; Phattharasupakun, Nutthaphon; et al.
Scientific Reports, 2017, 7, 17703
DOI : https://www.nature.com/articles/s41598-017-18108-0
Abstract: We design the Janus-like interlayer with two different functional faces for suppressing the shuttle of soluble lithium polysulfides (LPSs) in lithium-sulfur batteries (LSBs). At the front face, the conductive functionalized carbon fiber paper (f-CFP) having oxygen-containing groups i.e., -OH and -COOH on its surface was placed face to face with the sulfur cathode serving as the first barrier accommodating the volume expansion during cycling process and the oxygen-containing groups can also adsorb the soluble LPSs via lithium bonds. At the back face, a crystalline coordination network of [Zn(H2PO4)2(TzH)2]n (ZnPTz) was coated on the back side of f-CFP serving as the second barrier retarding the left LPSs passing through the front face via both physical confinement and chemical adsorption (i.e. Li bonding). The LSB using the Janus-like interlayer exhibits a high reversible discharge capacity of 1,416 mAh g−1 at 0.1C with a low capacity fading of 0.05% per cycle, 92% capacity retention after 200 cycles and ca. 100% coulombic efficiency. The fully charged LSB cell can practically supply electricity to a spinning motor with a nominal voltage of 3.0 V for 28 min demonstrating many potential applications.
4. A proton-hopping charge storage mechanism of ionic one-dimensional coordination polymers for high-performance supercapacitors
By: Phattharasupakun, Nutthaphon; Wutthiprom, Juthaporn; Kaenket, Surasak; et al.
Chemical Communications, 2017, 53, 11786-11789
DOI : https://pubs.rsc.org/en/content/articlelanding/2017/CC/C7CC07490E#!divAbstract
Abstract: A proton-conducting coordination polymer of anionic one-dimensional (1D) chains of Zn2+ phosphate and protonated imidazole with the formula of [Zn(HPO4)(H2PO4)2](ImH2)2 has been used as a novel supercapacitor material in aqueous electrolytes. This material stores charges via a proton-hopping mechanism.
5. High-performance energy storage of Ag-doped Co(OH)(2)-coated graphene paper: In situ electrochemical X-ray absorption spectroscopy
By: Suksomboon, Montakan; Khuntilo, Jakkrit; Kalasina, Saran; et al.
Electrochimica Acta, 2017, 252, 91-100
DOI : https://www.sciencedirect.com/science/article/pii/S0013468617318054?via%3Dihub
Abstract: Layered α-Co(OH)2 nanosheets have been widely investigated as one of the promising supercapacitor materials owing to its high theoretical specific capacitance (3,460 F g−1). However, their poor electrical conductivity, poor stability, and unclear charge storage mechanism limit their practical use in energy storage systems. To address the first two drawbacks of layered Co(OH)2, conductive silver nanoparticles (AgNPs) with a finely tuned loading content were for the first time incorporated to the layered Co(OH)2 structure coated on a flexible reduced graphene oxide (rGO)paper substrate. In situ electrochemical X-ray absorption spectroscopy (XAS) was also used to investigate the charge storage mechanism of AgNPs/Co(OH)2 electrode. It was found that the incorporation of AgNPs with an average diameter of <10 nm can significantly enhance the charge storage capacity of Co(OH)2 due to high electrical conductivity leading to fast charge transport. The charge storage mechanism of the Ag-doped α-Co(OH)2 energy storage cell is based on an intercalation/de-intercalation process (a battery-like behavior) and a physical adsorption of solvated ions (a supercapacitor-like behavior). The incorporation of AgNPs to the layered Co(OH)2 nanosheets in this work can overcome the current drawbacks of the Co(OH)2, which may lead to practical use as a hybrid energy storage device.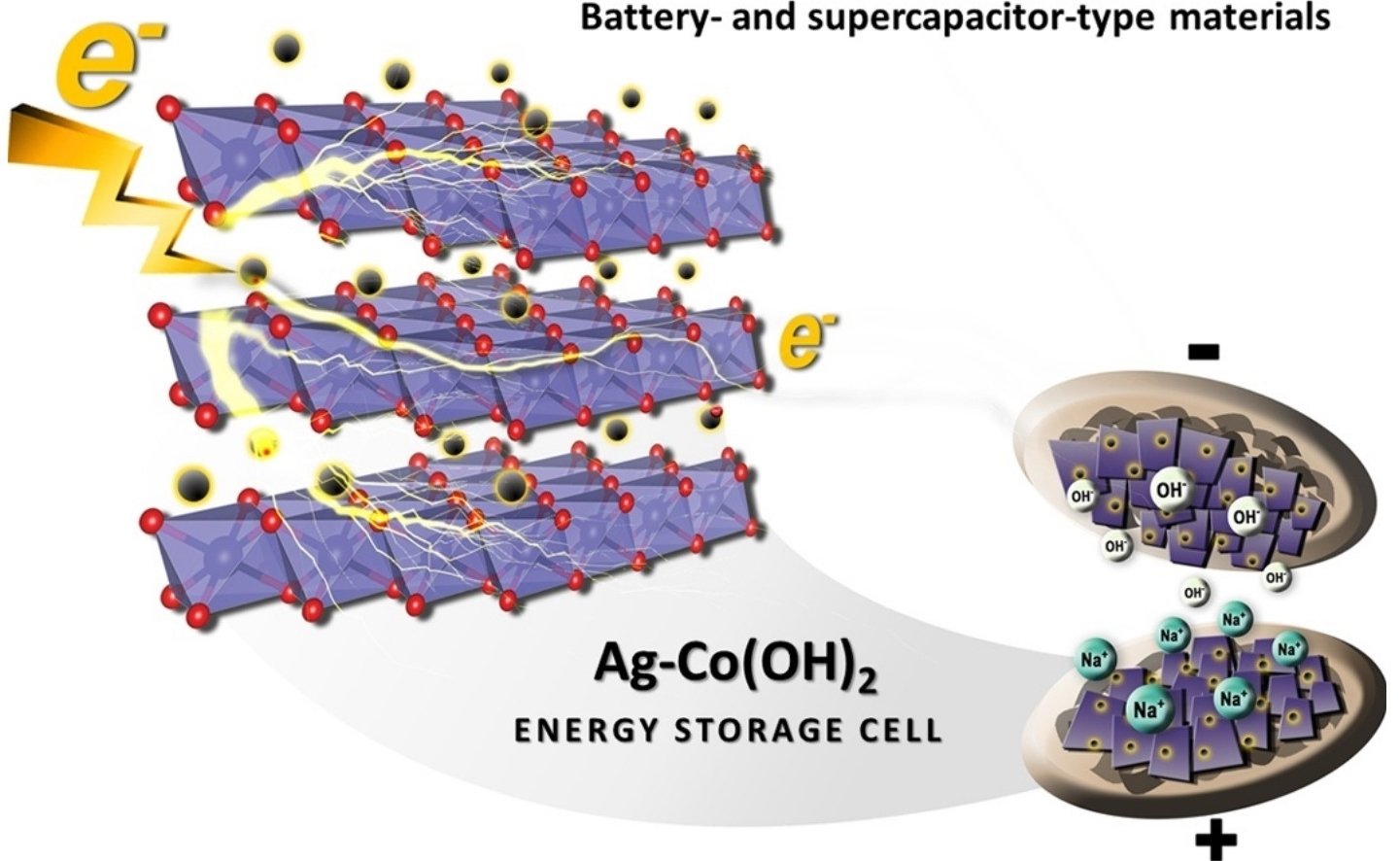

6. Antifungal activity of water-stable copper-containing metal-organic frameworks
By: Bouson, Supaporn; Krittayavathananon, Atiweena; Phattharasupakun, Nutthaphon; et al.
ROYAL SOCIETY OPEN SCIENCE, 2017, 4, 170654
DOI : https://pubmed.ncbi.nlm.nih.gov/29134075/
Abstract: Although metal-organic frameworks (MOFs) or porous coordination polymers have been widely studied, their antimicrobial activities have not yet been fully investigated. In this work, antifungal activity of copper-based benzene-tricarboxylate MOF (Cu-BTC MOF), which is water stable and industrially interesting, is investigated against Candida albicans, Aspergillus niger, Aspergillus oryzae and Fusarium oxysporum. The Cu-BTC MOF can effectively inhibit the growth rate of C. albicans and remarkably inhibit the spore growth of A. niger, A. oryzae and F. oxysporum. This finding shows the potential of using Cu-BTC MOF as a strong biocidal material against representative yeasts and moulds that are commonly found in the food and agricultural industries.
7. Strong adsorption of lithium polysulfides on ethylenediamine-functionalized carbon fiber paper interlayer providing excellent capacity retention of lithium-sulfur batteries
By: Kaewruang, Siriroong; Chiochan, Poramane; Phattharasupakun, Nutthaphon; et al.
Carbon, 2017, 123, 492-501
DOI : https://www.sciencedirect.com/science/article/pii/S0008622317307789?via%3Dihub
Abstract: Lithium-sulfur batteries (LSBs) are widely investigated since they have rather high theoretical capacity (1675 mAh g−1) and specific energy (∼2600 Wh kg−1). However, their poor capacity retention limits their practical use. Herein, sulfur-loaded activated carbon was used as the cathode of LSB. The functionalized carbon fiber paper (CFP) containing carboxyl and amide groups was used as an interlayer of LSB. With these designs of the LSB, it can provide excellent charge storage performance with high capacity retention. The LSB using ethylenediamine (EDA)-functionalized CFP interlayer exhibits an outstanding initial discharge capacity of 1800 mAh g−1 and 1472 mAh g−1 after the stable SEI formation with ca. 92% capacity retention and low degradation rate of 0.046% per cycle after 200 cycles at 1C. A strong adsorption capacity of polysulfide and amide functional group of EDA-functionalized CFP reduces the shuttle mechanism phenomenon. The density functional theory calculation shows that EDA-functionalized CFP can strongly bind high order polysulfides (Li2Sn, 4 ≤ n ≤ 8) when compared to other functional groups. Furthermore, the fully charged LSB cell with ca. 3.5 mg cm−2 sulfur loading content on the cathode can practically supply electricity to a nominal 3-V spinning motor for 25 min demonstrating the practical use of our as-fabricated LSB.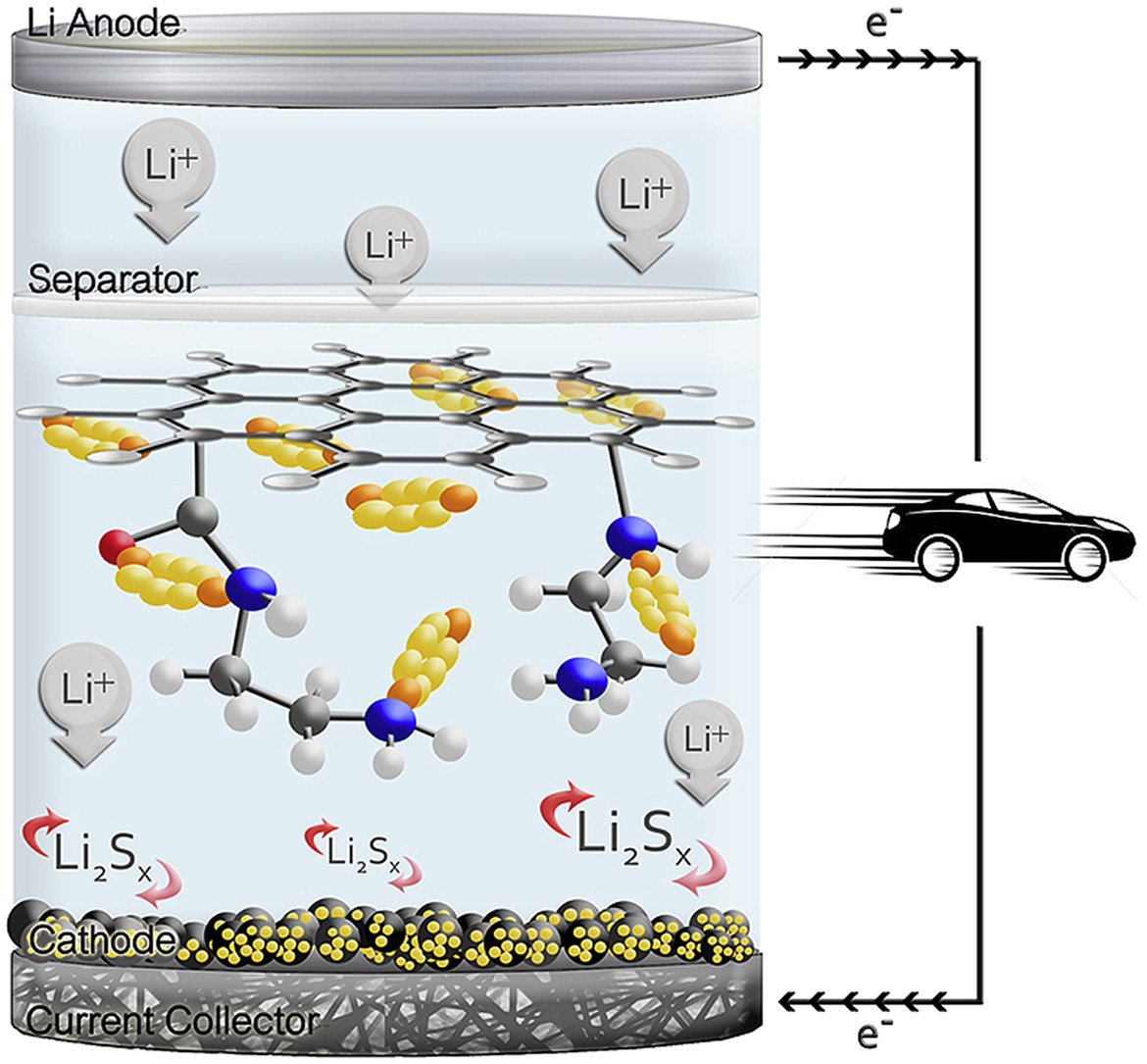

8. Collaborative design of Li-S batteries using 3D N-doped graphene aerogel as a sulfur host and graphitic carbon nitride paper as an interlayer
By: Wutthiprom, Juthaporn; Phattharasupakun, Nutthaphon; Khuntilo, Jakkrit; et al.
Sustainable Energy & Fuels, 2017, 1, 1759-1765
DOI : https://pubs.rsc.org/en/content/articlelanding/2017/SE/C7SE00291B#!divRelatedContent&articles
Abstract: Lithium–sulfur batteries (LSBs) have been widely investigated due to their high energy densities; however, their practical applications have still been limited by their poor cycling stability owing to the shuttle mechanism effect, volume expansion, soluble polysulfides, and the poor electrical conductivity of sulfur and Li2S. To address these issues, sulfur was loaded into a conductive 3D nitrogen-doped reduced graphene oxide aerogel (NGae) host with a finely tuned nitrogen doping content. In addition, an interlayer of graphitic carbon nitride coated on flexible and conductive carbon fiber paper (g-C3N4/CFP) was inserted between the cathode and the polymer separator to trap the soluble polysulfides. It was found that the as-fabricated LSB using the NGae host with 4.2% N doping content and the g-C3N4/CFP interlayer can provide a specific capacity of 1271 mA h g−1 at 0.1C with excellent stability over 400 cycles. The capacity fading is rather small (only 0.068% per cycle) while the coulombic efficiency is rather high (ca. 100%). This battery may be practically used in high-energy applications.
9. Charge storage performances and mechanisms of MnO2 nanospheres, nanorods, nanotubes and nanosheets
By: Tanggarnjanavalukul, Chan; Phattharasupakun, Nutthaphon; Kongpatpanich, Kanokwan; et al.
Nanoscale, 2017, 9, 13630-13639
DOI : https://pubs.rsc.org/en/content/articlelanding/2017/NR/C7NR02554H#!divAbstract
Abstract: Manganese dioxide (MnO2) has been widely used as an active material for high-performance supercapacitors due to its high theoretical capacitance, high cycling stability, low cost, and environmental friendliness. However, the effect of its crystallographic phase on charge storage performances and mechanisms is not yet clear. Herein, MnO2-based supercapacitors with different structures including nanospheres, nanorods, nanotubes, and nanosheets have been fabricated and investigated. Among such structures, δ-MnO2 nanosheets exhibit the highest specific capacitance of 194.3 F g−1 at 1 A g−1 when compared with other phases and shapes. The maximum specific energy of the δ-MnO2 nanosheet supercapacitor is 23.4 W h kg−1 at 971.6 W kg−1 and the maximum specific power is 4009.2 W kg−1 at 15.9 W h kg−1 with a capacity retention of 97% over 15 000 cycles. The δ-MnO2 nanosheet mainly stores charges via a diffusion-controlled mechanism at the scan rates of 10–100 mV s−1, whilst the α-MnO2 with different morphologies including nanospheres, nanorods, and nanotubes store charges via a non-faradaic or non-diffusion controlled process especially at fast scan rates (50–100 mV s−1). Understanding the charge storage performance and mechanism of the MnO2 nanostructures with different crystallographic phases and morphologies may lead to the further development of supercapacitors.
10. A new concept of charging supercapacitors based on the photovoltaic effect
By: Kalasina, Saran; Pattanasattayavong, Pichaya; Suksomboon, Montakan; et al.
CHEMICAL COMMUNICATIONS, 2017, 53, 709-712
DOI: https://www.rsc.org/suppdata/c6/cc/c6cc08131b/c6cc08131b1.pdf
Abstract: A significant enhancement in the areal capacitance of a Co(OH)2 supercapacitor charged and discharged under light illumination is clearly observed, with the capacitance about two-fold higher than that operated under dark conditions. This is because Co(OH)2 has an energy band gap of 2.85 eV and can absorb blue light and generate photoelectrons via the photovoltaic effect, leading to high current density.
11. Electrospinning of Carbon-Carbon Fiber Composites for High-Performance Single Coin-Cell Supercapacitors: Effects of Carbon Additives and Electrolytes
By: Suktha, Phansiri; Sawangphruk, Montree
INDUSTRIAL & ENGINEERING CHEMISTRY RESEARCH, 2017, 56, 10078–10086
DOI : https://pubs.acs.org/doi/10.1021/acs.iecr.7b02797
Abstract: Carbon nanofibers incorporated with other carbon additives, i.e. acetylene black (ACB), hollow carbon sphere (HCS), and reduced graphene oxide sheets (rGO), were successfully produced by an electrospinning process. The influence of carbon additives was evaluated through the electrochemical performance of carbon-based nanofiber supercapacitors. The carbon additives with high sp2 content not only enhanced specific surface area but also improved electrical conductivity of the carbon nanofibers. A finely tuned 1 wt % ACB loaded to the carbon nanofibers provided a specific surface area of 116 m2 g–1, a specific capacitance of 209 F g–1 at 2 mA per cell, a specific energy of 14.2 Wh kg–1, a maximum specific power of 8.3 kW kg–1, and >99% capacity retention over 10 000 cycles in 1 M H2SO4 aqueous electrolyte. The carbon fiber composites here may be practically used in supercapacitors.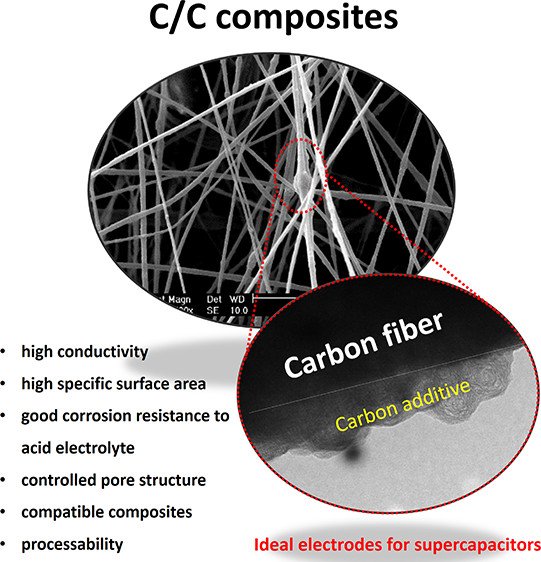

12. Insight into charge storage mechanisms of layered MnO2 nanosheets for supercapacitor electrodes: In situ electrochemical X-ray absorption spectroscopy
By: Iamprasertkun, Pawin; Tanggarnjanavalukul, Chan; Krittayavathananon, Atiweena; et al.
Electrochimica Acta, 2017, 249, 26-32
DOI : https://www.sciencedirect.com/science/article/pii/S0013468617316298?via%3Dihub
Abstract: Manganese dioxide (MnO2) nanostructures have been widely used as the supercapacitor electrode. However, their charge storage mechanism has not yet been fully understood. Herein, the charge storage mechanism of layered MnO2 nanosheets has been investigated. It was found that they store charges via three processes: electrochemical double layer capacitance (EDLC), surface redox reaction, and intercalation/de-intercalation. The EDLC of the layered MnO2 is equal to 71.6 F g−1 at all scan rates used (10–100 mV s−1) without a diffusion limit. The surface redox contribution owing to the chemisorption of Na+ on the surface of MnO2 nanosheets is 30.8, 18.0, 20.0, 17.0, 13.5 F g−1 at 10, 25, 50, 75, and 100 mV s−1, respectively. The intercalation contribution thanks to the solvated Na+ insertion into the crystalline bulk structure of layered MnO2 is 96.4, 57.1, 38.6, 30.5, and 25.3 F g−1 at 10, 25, 50, 75, 100 mV s−1, respectively. The surface redox reaction and intercalation are still under diffusion limit. Understanding the charge storage mechanism/contribution of MnO2 nanosheets may lead to further development of these materials for practical applications.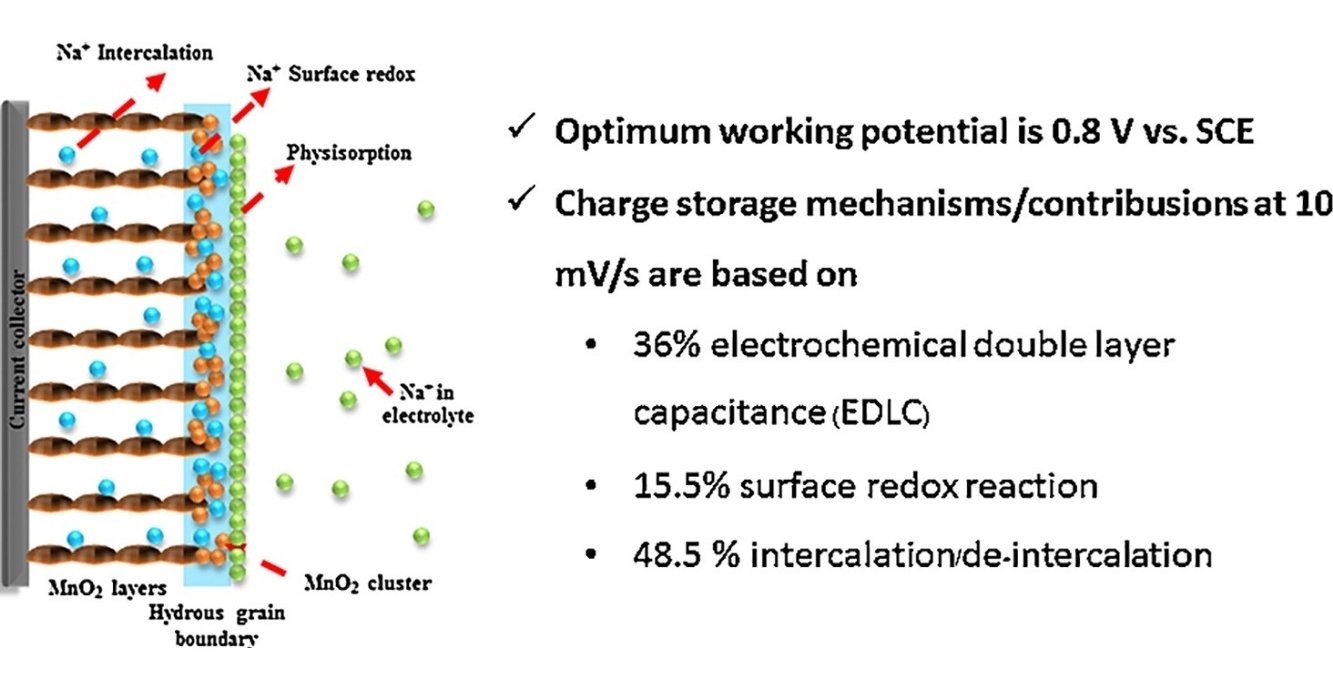

13. Improving Single-Carbon-Nanotube-Electrode Contacts Using Molecular Electronics
By: Krittayavathananon, Atiweena; Ngamchuea, Kamonwad; Li, Xiuting; et al.
JOURNAL OF PHYSICAL CHEMISTRY LETTERS, 2017, 8, 3908-3911
DOI: https://pubs.acs.org/doi/10.1021/acs.jpclett.7b01771
Abstract: We report the use of an electroactive species, acetaminophen, to modify the electrical connection between a carbon nanotube (CNT) and an electrode. By applying a potential across two electrodes, some of the CNTs in solution occasionally contact the electrified interface and bridge between two electrodes. By observing a single CNT contact between two microbands of an interdigitated Au electrode in the presence and absence of acetaminophen, the role of the molecular species at the electronic junction is revealed. As compared with the pure CNT, the current magnitude of the acetaminophen-modified CNTs significantly increases with the applied potentials, indicating that the molecule species improves the junction properties probably via redox shuttling.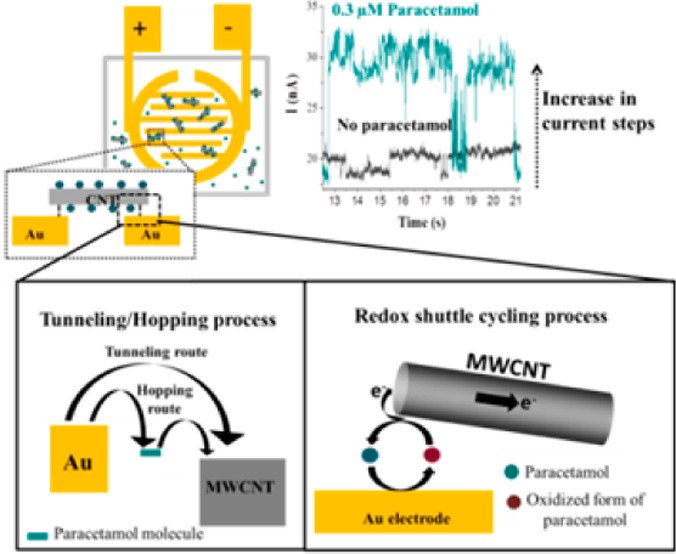

14. Turning Carbon Black to Hollow Carbon Nanospheres for Enhancing Charge Storage Capacities of LiMn2O4, LiCoO2, LiNiMnCoO2, and LiFePO4 Lithium-Ion Batteries
By: Wutthiprom, Juthaporn; Phattharasupakun, Nutthaphon; Sawangphruk, Montree
ACS OMEGA, 2017, 2, 3730-3738
DOI: https://pubs.acs.org/doi/10.1021/acsomega.7b00763
Abstract: Carbon black nanospheres were turned to hollow carbon nanospheres (HCNs) and were used as the conductive additive in the cathodes of Li-ion batteries (LIBs). The results show that 10 wt % HCN added to the LIB cathodes, such as LiMn2O4, LiCoO2, LiNiMnCoO2, and LiFePO4, can provide significantly higher specific capacity than those using spherical carbon black. For example, a specific capacity of the LiMn2O4/HCN/PVDF cathode at 80:10:10 wt % with a bulk electrical conductivity of 1.07 Ω cm–2 is 125 mA h g–1 at 0.1 C from 3.0 to 4.3 V versus Li+/Li, which is 3.85-fold higher than that using Super P. The stability tested at 1 C remains over 95% after 800 charge/discharge cycles with 100% Coulombic efficiency. Replacing the present carbon black conductive additive with HCN in this work may be one of the best choices to increase the charge storage performance of LIBs rather than only focusing on the development of active cathode materials.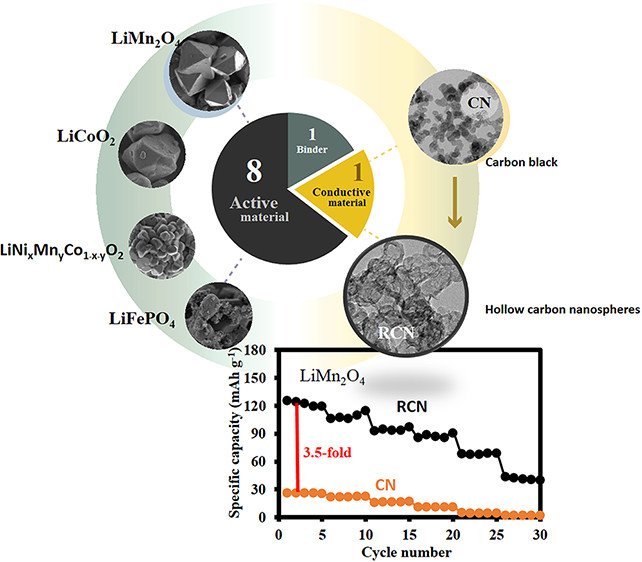

15. High-performance supercapacitors of carboxylate-modified hollow carbon nanospheres coated on flexible carbon fibre paper: Effects of oxygen-containing group contents, electrolytes and operating temperature
By: Phattharasupakun, Nutthaphon; Wutthiprom, Juthaporn; Suktha, Phansiri; et al.
Electrochimica Acta, 2017, 238, 64-73
DOI: https://www.sciencedirect.com/science/article/pii/S001346861730717X?via%3Dihub
Abstract: Although functionalized carbon-based materials have been widely used as the supercapacitor electrodes, the optimum contents of the functional groups, the charge storage mechanisms, and the effects of electrolytes and operating temperature have not yet been clearly investigated. In this work, carboxylate-modified hollow carbon nanospheres (c-HCN) with different functional group contents synthesized by an oxidation process of carbon nanospheres with nitric acid were coated on flexible carbon fibre paper and used as the supercapacitor electrodes. An as-fabricated supercapacitor of the c-HCN with a finely tuned 6.2 atomic % of oxygen of the oxygen-containing groups in an ionic liquid electrolyte exhibits a specific capacitance of 390 F g−1, a specific energy of 115 Wh kg−1, and a maximum specific power of 13548 W kg−1 at 70 °C. The charge storage mechanism investigated is based on the chemical adsorption of the ionic liquid electrolyte on the c-HCN electrode. This process is highly reversible leading to high capacity retention. The supercapacitor in this work may be practically used in many high energy and power applications.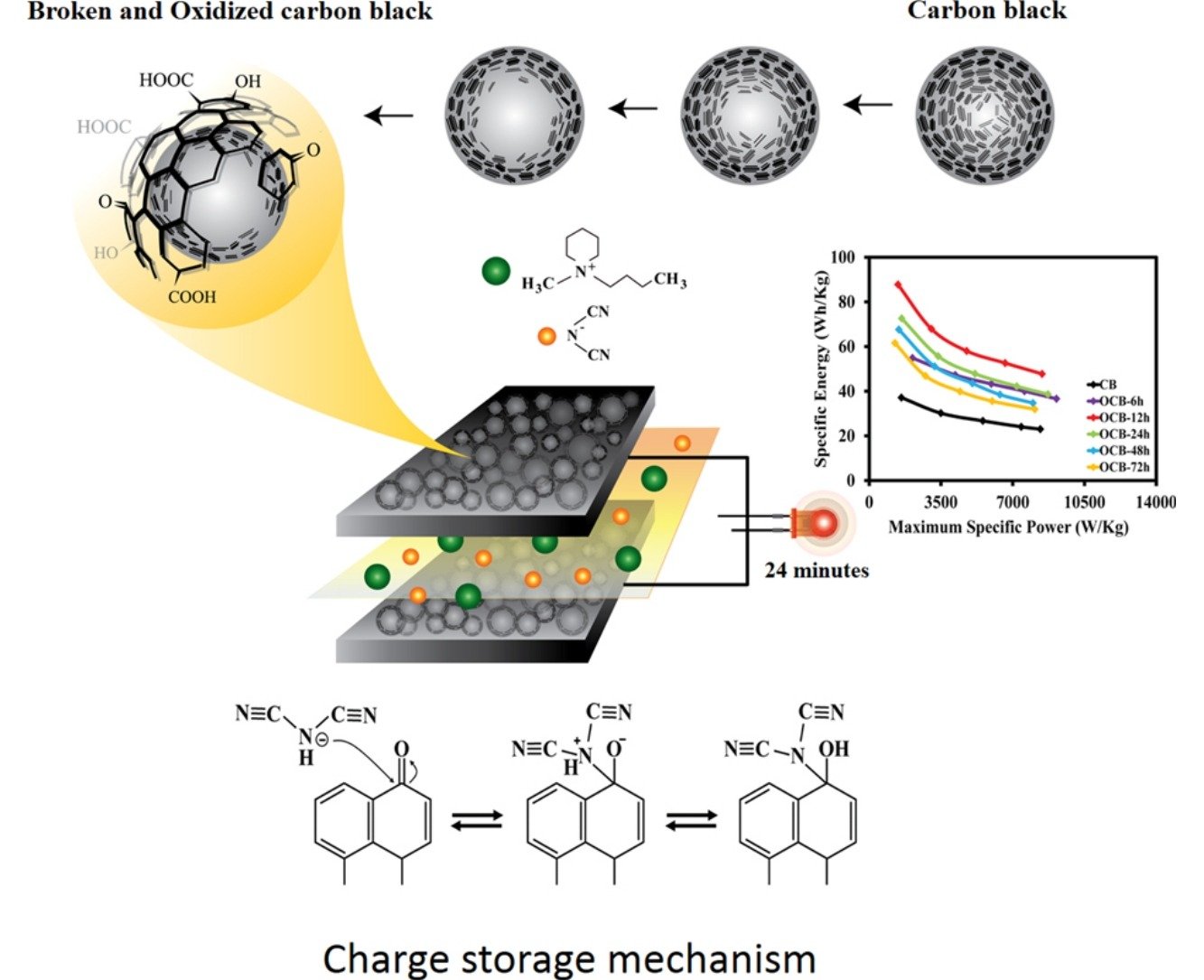

16. Core-double shell sulfur@carbon black nanosphere@oxidized carbon nanosheet composites as the cathode materials for Li-S batteries
By: Chiochan, Poramane; Phattharasupakun, Nutthaphon; Wutthiprom, Juthaporn; et al.
Electrochimica Acta, 2017, 237, 78-86
DOI: https://www.sciencedirect.com/science/article/pii/S0013468617307090?via%3Dihub
Abstract: Although lithium-sulfur batteries (LSBs) have rather high capacity and specific energy, they are facing many drawbacks, e.g. low electrical conductivity of sulfur (S8) and lithium sulfide (Li2S), low capacity retention, large volume expansion of lithium sulfide in full lithiation state and dissolution of intermediate lithium polysulfides into the electrolytes. In order to address some drawbacks of the LSBs, here, sulfur was encapsulated by carbon black nanospheres (CN) and oxidized carbon nanosheet (OCN) with a sheet-like morphology by a mechanofusion and a colloidal coating techniques, respectively. Core-double shell S@CN@OCN composites were used as the cathode materials for Li-S batteries. The first CN shell can reduce the volume expansion of the sulfur during the charging process and also enhance the electrical conductivity. The second OCN shell can accommodate the volume expansion of the sulfur and decrease the “redox shuttle effect”. The LSB using the core-double shell S@CN@OCN cathode can provide the first and second discharge specific capacities of 1,313 and 1,279 mAh g−1 at 0.1C, respectively. In addition, the stability of this LIB tested at 1C over 400 cycles provides 77% capacity retention and 98% coulombic efficiency. This newly designed core-double shell S@CN@OCN structure may be the promising cathode of LSBs leading to the practical applications.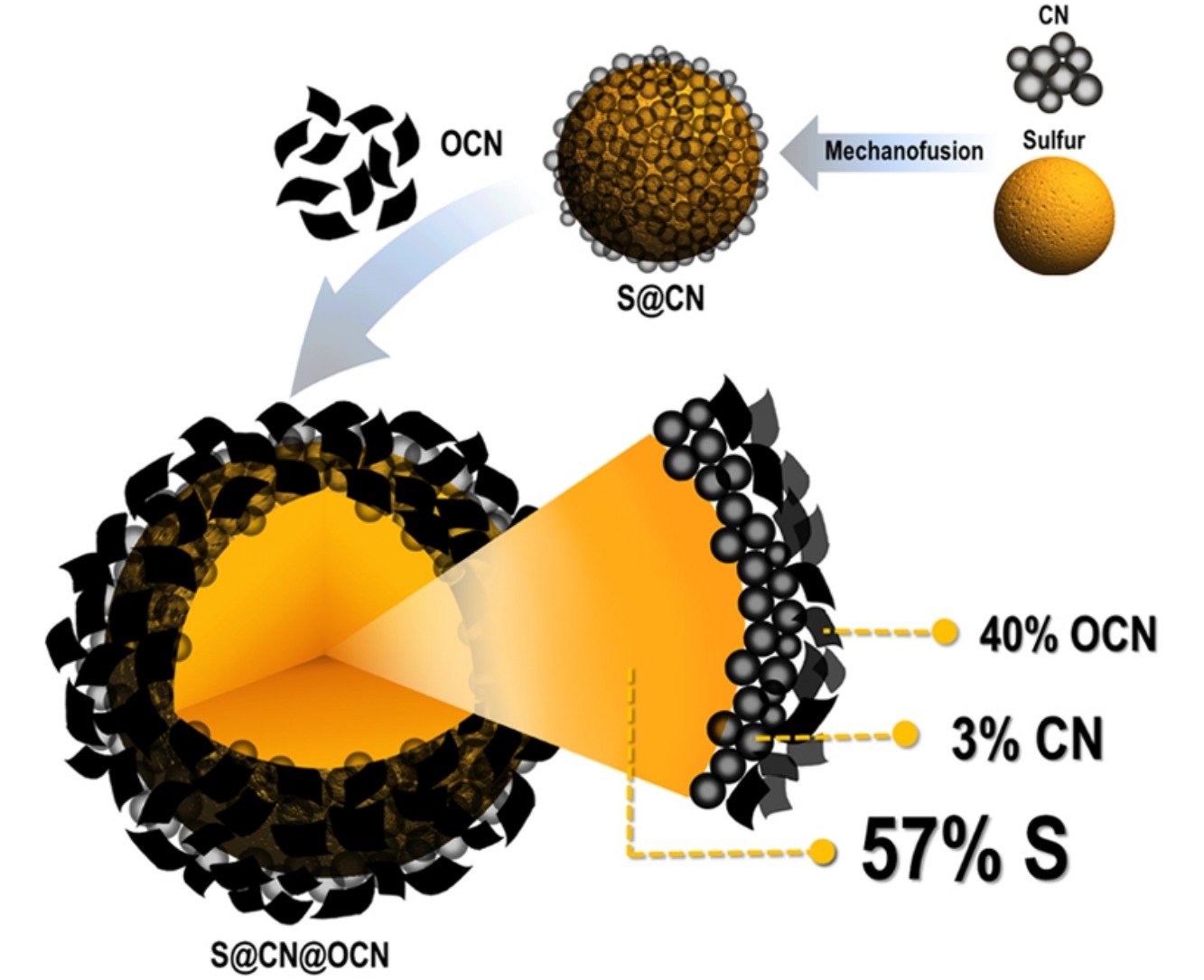

17. Hybrid Energy Storage of Ni(OH)(2)-coated N-doped Graphene Aerogel//N-doped Graphene Aerogel for the Replacement of NiCd and NiMH Batteries
By: Sirisinudomkit, Pichamon; Iamprasertkun, Pawin; Krittayavathananon, Atiweena; et al.
SCIENTIFIC REPORTS Volume: 7 Article Number: 1124 Published online: 25 April 2017
DOI: https://www.nature.com/articles/s41598-017-01191-8.pdf
Abstract: Although Nickel–Cadmium (NiCd) and Nickel–metal hydride (NiMH) batteries have been widely used, their drawbacks including toxic Cd and expensive La alloy at the negative electrodes, low energy density (40–60 Wh/kg for NiCd and 140–300 Wh/L for NiMH), low power density (150 W/kg for NiCd and 1000 W/kg for NiMH), and low working potential (1.2 V) limit their applications. In this work, Cd and La alloy were replaced with N-doped reduced graphene oxide aerogel (N-rGOae) providing a hybrid energy storage (HES) having the battery and supercapacitor effects. The HES of Ni(OH)2-coated N-rGOae//N-rGOae provides 1.5 V, a specific energy of 146 Wh/kg, a maximum specific power of 7705 W/kg, and high capacity retention over 84.6% after 5000 cycles. The mass change at the positive electrode during charging/discharging is 8.5 µg cm−2 owing to the insertion/desertion of solvated OH− into the α-Ni(OH)2-coated N-rGOae. At the negative electrode, the mass change of the solvated K+, physically adsorbed/desorbed to the N-rGOae, is 7.5 μg cm−2. In situ X-ray absorption spectroscopy (XAS) shows highly reversible redox reaction of α-Ni(OH)2. The as-fabricated device without using toxic Cd and expensive La alloy has a potential as a candidate of NiCd and NiMH.
18. Hybrid energy storage of battery-type nickel hydroxide and supercapacitor-type graphene: redox additive and charge storage mechanism
By: Sirisinudomkit, Pichamon; Iamprasertkun, Pawin; Krittayavathananon, Atiweena; et al.
SUSTAINABLE ENERGY & FUELS, 2017, 7, 275-279
DOI: https://pubs.rsc.org/en/content/articlelanding/2017/SE/C7SE00052A#!divAbstract
Abstract: Herein, hybrid energy storages (HESs) of battery-type Ni(OH)2 and supercapacitor-type electrochemically reduced graphene oxide (ERGO) were fabricated using potassium ferricyanide (K3Fe(CN)6) as a redox additive in KOH electrolyte for high specific energy and power applications. The as-fabricated HES of Ni(OH)2//ERGO in a single coin cell (CR2016) size in 4 mM K3[Fe(CN)]6 in 1 M KOH provides a wide working voltage up to 1.6 V and exhibits a maximum specific energy of 85 W h kg−1 at the specific power of 726 W kg−1 with a high capacity retention over 88% after 10 000 cycles, while the HES in 1 M KOH provides a lower maximum specific energy of 61 W h kg−1. A Fe(CN)63−/Fe(CN)64− redox couple has a great electrochemical reversibility in nature since Fe(CN)63− can obtain electrons from Ni(OH)2 through the reduction process and Fe(CN)64− can donate electrons to NiOOH for the oxidation process. The HES reported herein may be practically used for high energy applications.
19. Decoration of graphene oxide nanosheets with amino silane-functionalized silica nanoparticles for enhancing thermal and mechanical properties of polypropylene nanocomposites
By: Klunbud, Panupong; Suktha, Phansiri; Sawangphruk, Montree
JOURNAL OF APPLIED POLYMER SCIENCE, 2017, 134, 44382
DOI: https://onlinelibrary.wiley.com/doi/full/10.1002/app.44382
Abstract: Graphene oxide nanosheets were decorated by amino‐silane modified silica nanoparticles. An electrostatic interaction between the negative charge of oxygen‐containing groups of graphene oxide and the positive charge of amino‐silane functional groups on the surface of silica nanoparticles plays a major role for the interfacial interaction of these two materials. The hybrid material was then used as a reinforcement in polypropylene (PP) composite. The increasing tensile strength at yield, tensile, and flexural modulus of the PP composite at a graphene oxide‐ amino‐silane silica loading content of 20 wt % are about 24.81, 55.52, and 30.35%, respectively, when compared with those of PP. It is believed that GO assists the dispersion of SiO2 nanoparticles to the polymer matrix because of its unique structure having hydrophilicity due to its oxygen functional groups and hydrophobicity owing to its backbone graphitic carbon structure. This hybrid material may also be used as the reinforcement in other polyolefins.
20. Charge storage mechanisms of electrospun Mn3O4 nanofibres for high-performance supercapacitors
By: Suktha, Phansiri; Phattharasupakun, Nutthaphon; Dittanet, Peerapan; et al.
RSC Advances, 2017, 7, 9958-9963
DOI: https://pubs.rsc.org/en/content/articlelanding/2017/ra/c6ra28499j
Abstract: Mixed oxidation states of manganese oxides are widely used as the electrodes in supercapacitors due to their high theoretical pseudocapacitances. However, their charge storage mechanisms are not yet fully understood. In this work, the charge storage mechanism of Mn3O4 or Mn2+(Mn3+)2O4 nanofibres was investigated using a synchrotron-based X-ray absorption spectroscopy (XAS) technique and an in situ electrochemical quartz crystal microbalance (EQCM). The average oxidation state of the Mn in the as-synthesized Mn3O4 is +2.67. After the first charge, the average oxidation states of Mn at the positive and negative electrodes are +2.61 and +2.38, respectively. The significant change in the oxidation state of Mn at the negative electrode is due to phase transformation of Mn3O4 to NaδMnOx·nH2O. Meanwhile, the charge storage mechanism at the positive electrode mainly involves the adsorption of counter ions or solvated SO42−. After the first discharge, the calculated Mn average oxidation numbers are +2.51 and +2.53 at the positive and negative electrodes, respectively. At the negative electrode, the solvated Na+ is desorbed from the electrode surface. At the same time, the solvated SO42− is desorbed from the positive electrode. The mass change of solvated Na+ during charging/discharging is ca. 80 ng per cm2 of the Mn3O4 electrode. A symmetric supercapacitor constructed from Mn3O4 nanofibres in 0.5 M Na2SO4 provides a working potential of 1.8 V, a specific energy of 37.4 W h kg−1 and a maximum specific power of 11.1 kW kg−1 with 98% capacity retention over 4500 cycles. The understanding of the charge storage mechanism of the mixed oxidation states of Mn2+(Mn3+)2O4 presented in this work could lead to further development of metal oxide-based pseudocapacitors.
21. Chemical Adsorption and Physical Confinement of Polysulfides with the Janus-faced Interlayer for High-performance Lithium-Sulfur Batteries
By: Chiochan, Poramane; Kaewruang, Siriroong; Phattharasupakun, Nutthaphon; et al.
Scientific Reports, 2017, 7, 17703
DOI: https://www.nature.com/articles/s41598-017-18108-0
Abstract: We design the Janus-like interlayer with two different functional faces for suppressing the shuttle of soluble lithium polysulfides (LPSs) in lithium-sulfur batteries (LSBs). At the front face, the conductive functionalized carbon fiber paper (f-CFP) having oxygen-containing groups i.e., -OH and -COOH on its surface was placed face to face with the sulfur cathode serving as the first barrier accommodating the volume expansion during cycling process and the oxygen-containing groups can also adsorb the soluble LPSs via lithium bonds. At the back face, a crystalline coordination network of [Zn(H2PO4)2(TzH)2]n (ZnPTz) was coated on the back side of f-CFP serving as the second barrier retarding the left LPSs passing through the front face via both physical confinement and chemical adsorption (i.e. Li bonding). The LSB using the Janus-like interlayer exhibits a high reversible discharge capacity of 1,416 mAh g−1 at 0.1C with a low capacity fading of 0.05% per cycle, 92% capacity retention after 200 cycles and ca. 100% coulombic efficiency. The fully charged LSB cell can practically supply electricity to a spinning motor with a nominal voltage of 3.0 V for 28 min demonstrating many potential applications.