1. Recycled Additive Manufacturing Feedstocks for Fabricating High Voltage, Low-Cost Aqueous Supercapacitors
Phatsawit Wuamprakhon, Robert D. Crapnell, Evelyn Sigley, Nicholas J. Hurst, Rhys J. Williams, Montree Sawangphruk, Edmund M. Keefe, Craig E. Banks
Advanced Sustainable Systems, 2022
DOI: https://doi.org/10.1002/adsu.202200407
Abstract: The first recycled conductive poly(lactic acid) (PLA) filament derived from post-industrial waste sources for additive manufacturing (AM) is reported herein, presenting a paradigm shift in plastic waste recycling, AM filament production, and AM energy storage architectures. Filaments utilizing a base of recycled PLA, carbon black (CB) as a conductive filler, and polyethylene glycol (PEG) as a plasticizer are used to produce aqueous AM symmetric supercapacitor platforms that can reach capacitance values 75 times higher than commercially available conductive PLA filaments. Furthermore, through the rapid prototyping capabilities of AM and GCode modification, it is seen that changing the electrode architecture from solid to a mesh with additional inter-layer spacing is able to further enhance electrode performance by 3.5 times due to improvements in the surface area, ion accommodating capabilities and faster ion diffusion. The symmetric full cell device is capable of delivering 7.82 mF cm−2, 4.82 µWh cm−2, and 433.32 µW cm−2 of capacitance, energy, and power density, respectively. Moreover, the material cost is £0.15 per electrode. This work represents a new direction for plastic waste recycling, in which low-value recycled base products can be manufactured into high-value end products in their second cycles.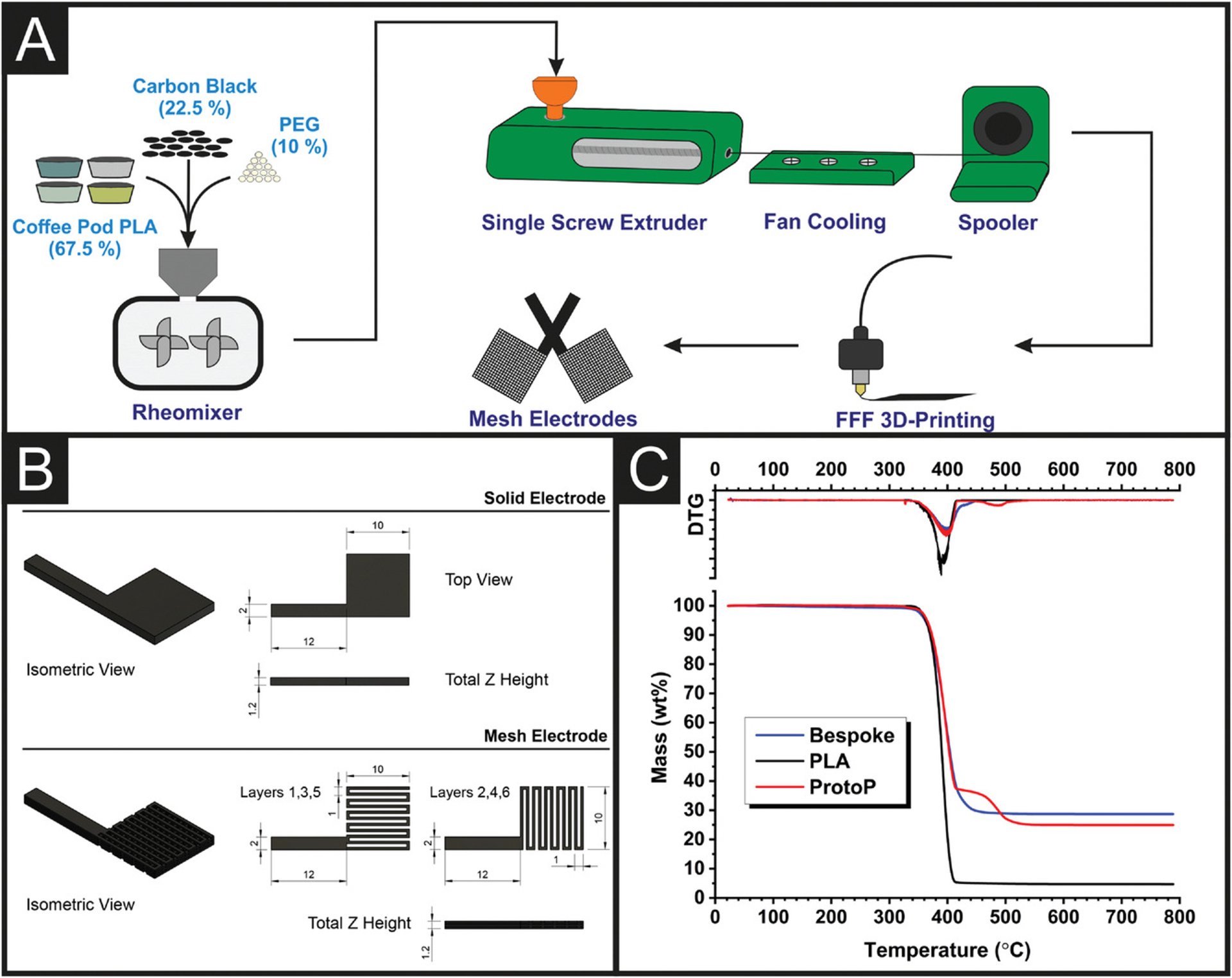

2. Size Selection and Size-Dependent Optoelectronic and Electrochemical Properties of 2D Titanium Carbide (Ti3C2Tx) MXene
Kanit Hantanasirisakul, Treepat Chantaurai, Apichanont Limsukhon, Praeploy Chomkhuntod, Phuri Poprom, Montree Sawangphruk
Advanced Materials Interfaces, 2022, 2201457
DOI: https://doi.org/10.1002/admi.202201457
Abstract: 2D materials produced via liquid-phase exfoliation commonly exhibit broad size and thickness distributions, where size selection is required to obtain nanosheets with desired dimensions and properties. Here, size selection of 2D Ti3C2Tx MXene is systematically performed using liquid cascade centrifugation (LCC) and sedimentation-based separation (SBS) techniques. Based on statistical atomic force and electron microscopy analyses, it is found that the SBS technique offers stronger separation power compared to the LCC technique with an expense of lower yield. Moreover, predominantly monolayer MXene flakes are obtained after centrifugation at low speeds (≤ 3000 rpm) for 15 min for both techniques. Films fabricated from large MXene flakes with the average flake length of 4.2 µm show high electrical figure of merit of 7.8 and improved rate performance as a cathode in Zn-ion capacitor. This work serves as a guideline for choosing size selection techniques and conditions for MXene processing to obtain flakes with certain sizes and desired properties.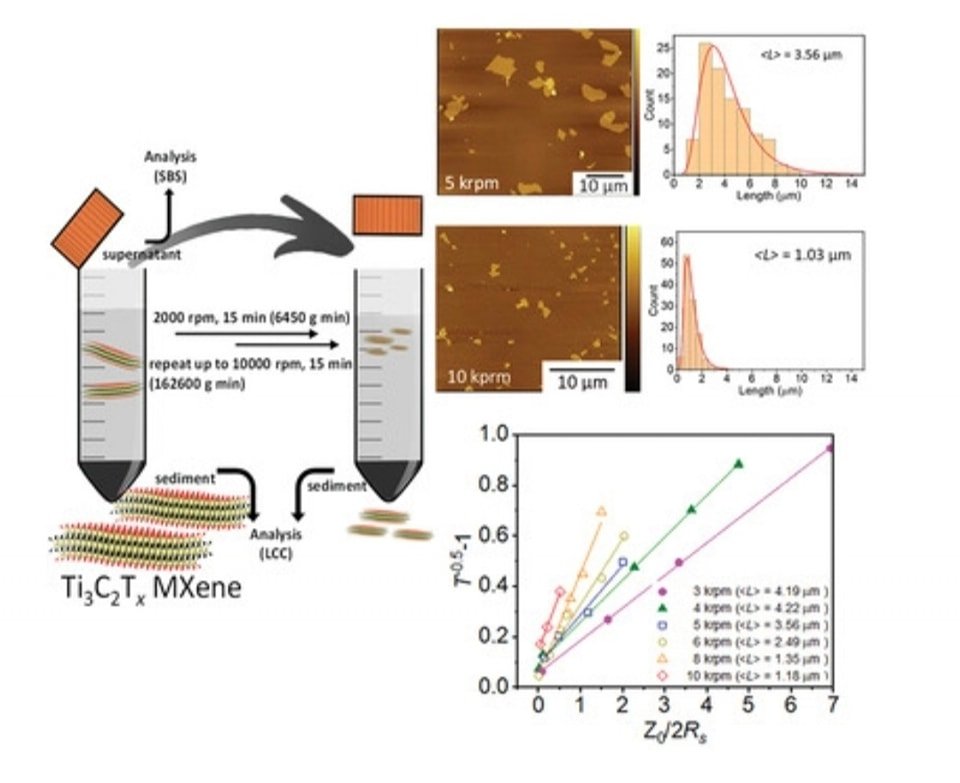

3. Twin boundaries boost the hydrogen evolution reaction on the solid solution of nickel and tungsten
Jiuchao Tang, Jingjing Niu, Chengwu Yang, Saravanan Rajendran, Yongpeng Lei, Montree Sawangphruk, Xinyu Zhang, Jiaqian Qin
Fuelมม 2022, 330, 125510
DOI: https://doi.org/10.1016/j.fuel.2022.125510
Abstract: The development of cost-effective electrocatalysts to produce high-purity hydrogen by electrochemical water splitting is critical to addressing resource and environmental problems. Theoretically, NiW materials are considered to be an excellent HER electrocatalyst. However, current NiW catalysts for HER are not satisfactory. Herein, the NiW solid solution with twin boundaries is successfully fabricated by facile electrodeposition on copper sheet. As compared with Ni, the NiW-0.6A catalyst shows enhanced HER activity in 1 M KOH with the overpotential of 98 mV at 10 mA/cm2. Besides, NiW-0.6A exhibits excellent stability for 100 h at 20 mA/cm2. The excellent HER performance of NiW-0.6A may be attributed to the intrinsic catalytic activity and the defective twin boundaries exposing many active sites. This work could provide feasible suggestions for the design of efficient and high-performance NiW electrocatalysts for HER process..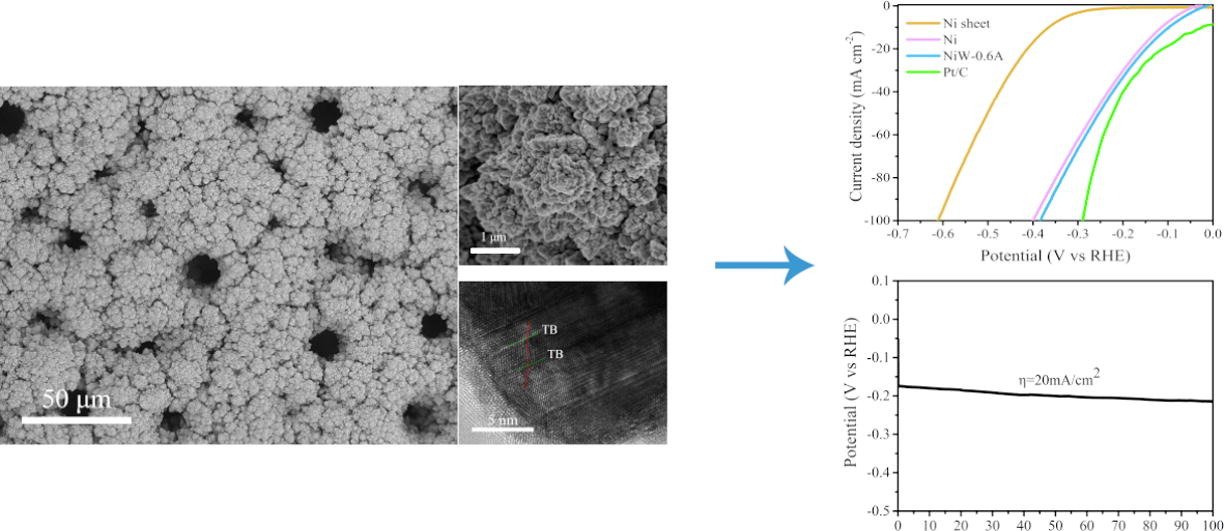

4. Reducing Intrinsic Drawbacks of Ni-rich Layered Oxide Cathode Materials with a Dry Coating Concept of Quasi-solid Nanomaterials towards High-performance Cylindrical Li-ion
Poramane Chiochan, Chonticha Jangsan, Nichakarn Anansuksawat, Kan Homlamai, Nattanon Joraleechanchai, Worapol Tejangkura, Montree Sawangphruk
Journal of The Electrochemical Society
DOIซ https://iopscience.iop.org/article/10.1149/1945-7111/aca2e2/meta
Abstract: Although Ni-rich layered oxide cathode materials of Li-ion batteries can provide high energy density, their performance degradation over long cycling and safety hazard due to their intrinsic property issues limit their practical long-term applications. Herein, we introduce a concept based on Ni-rich NMC811 core@quasi-solid shell structure. The Li-rich quasi-solid shell material was prepared by infusing 2 M LiTFSI in [EMIM][TFSI] into a whole pore of Al2O3 nanoparticles delivering a high ionic conductivity (2.8 × 10−4 S cm−1) at room temperature (25 °C). Then the shell material with a thickness of ca. 200 nm below a "Play Dough-like" state was coated on NMC811 using a green and scalable mechanofusion process. The 18650 cylindrical Li-ion battery cells using the core-shell cathode and the graphite anode at a pilot-plant manufacturing scale exhibit considerable high-rate capability compared to the pristine NMC811, especially at a high C-rate. The post-mortem analysis demonstrated that with the thick semi-solid shell there is no transition metal dissolution. Also, the battery cells retained a high discharge capacity after long-term cycling without any safety hazards. We believe that the semi-solid encapsulation in this work may be useful for next-generation high-energy Ni-rich Li-ion batteries.
5. Li-ion batteries of Ni-rich lithium nickel cobalt aluminium oxide coupled with high-energy lithiophilic anode
Poramane Chiochan, Phansiri Suktha, Nutthaphon Phattharasupakun, Salatan Duangdangchote, Montakan Suksomboon, Worapol Tejangkura, Montree Sawangphruk
SCIENCE SOCIETY THAILAND, 2022, 076, 1513-1874
DOI: http://www.scienceasia.org/acconline/076-2021-7100.pdf
Abstract: Herein, highly dispersed 10-nm lithiophilic silver nanoparticles (AgNPs) were synthesized and decorated on 3D graphene aerogel supporting materials. The prelithiated AgNPs/3D graphene aerogel exhibits a high specific capacity of 1589 mAh/g at 0.1 A/g with a high initial coulombic efficiency (ICE) of ca. 93% and a long cycling stability over 500 cycles. A Li-ion battery cell using the prelithiated AgNPs/3D graphene aerogel with a finely tuned Ag content of 0.52 at. % as the anode and the Ni-rich LiNi0.88Co0.09Al0.03O2 as the cathode exhibits a high energy density of ∼290 Wh/kg at 0.1 C. This new anode material may be a useful high-energy anode for next-generation Li-ion batteries.
6. Graphene Aerogel-encapsulated Silicon Nanoparticles Mechanofused on Graphite without Prelithiation for Cylindrical Ni-rich NMC811 Li-ion Batteries
Ketsuda Kongsawatvoragul, Panyawee Bunyanidhi, Chonticha Jangsan, Worapol Tejangkura, Montree Sawangphruk
Journal of The Electrochemical Society, 2022, 169, 090524
DOI: https://iopscience.iop.org/article/10.1149/1945-7111/ac91ad/meta
Abstract: Silicon (Si), one of the promising anodes, provides a high theoretical specific capacity of ca. 3500 mAh g−1 at room temperature. It experiences many drastic issues, such as cost-effectiveness, large volume expansion, and unstable thick solid–electrolyte interfaces (SEI), leading to poor cycling stability. A small amount of Si has recently been added to graphite and used as the anode for commercial Li-ion batteries. Nevertheless, the intrinsic issues of Si still occur. Herein, we encapsulated Si nanoparticles with reduced graphene oxide (RGO) aerogel and graphite to obtain Si-RGO@Graphite using a dry surface coating technique so-called mechanofusion. This technique enhances the strong binding between these materials. We also demonstrated the practical use of the as-prepared Si-RGO@Graphite (9.9:0.1:90.0 wt% of Si:RGO:Graphite) anode coupling with Ni-rich NMC811 cathode at a 18650 cylindrical cell level. In this attempt, we avoid using an expensive vacuum-required prelithiation process, which currently inhibits the practical and commercial use of the Si-based anode. We believe this new composite material may be useful for high-energy LIBs in the future.
7. Inhibition of Gas-evolved Electrolyte Decomposition in Cylindrical Li-ion Battery Cells of Ni-rich Layered Oxide with A Dry Coating Process without Post Thermal Annealing
Krisara Srimanon, Selvamani Vadivel, Montree Sawangphruk
Journal of Power Sources, 2022, 550, 232150
DOI: https://doi.org/10.1016/j.jpowsour.2022.232150
Abstract: Although Ni-rich layered oxide materials widely used as the cathode of lithium-ion batteries (LIBs) possess several interesting properties e.g., high energy density, they encounter severe capacity degradation and intrinsic poor thermal stability concerning the Ni-concentration. Herein, a core-shell-like architecture has been achieved by employing the cost-effective dry particle fusion method without an energy-consuming thermal annealing process with alumina (Al2O3) nanoparticles over LiNi0.8Mn0.1Co0.1O2 or NMC811 and resulting in an intact layer with an average shell thickness of 150–200 nm. Such NMC811@ Al2O3 core-shell exhibits excellent cycling stability compared with pristine NMC811. The chemical lithium diffusion coefficient, as calculated from a galvanostatic intermittent titration technique (GITT) infers that the introduction of Al2O3 coating slightly reduces the lithium diffusivity; however, it limits the active material dissolution, as perceived from post-ICP-OES analysis of the counter electrode. Besides, such modified systems exhibit a significant delay in onset potential related to surface layer decomposition and lattice oxygen release. Therefore, the present investigation concludes that the Al2O3 shell in the NMC811@ Al2O3 not only offers superior electrochemical performance but also exhibits good thermal stability. It could achieve thru a cost-effective and scalable dry-fusion method.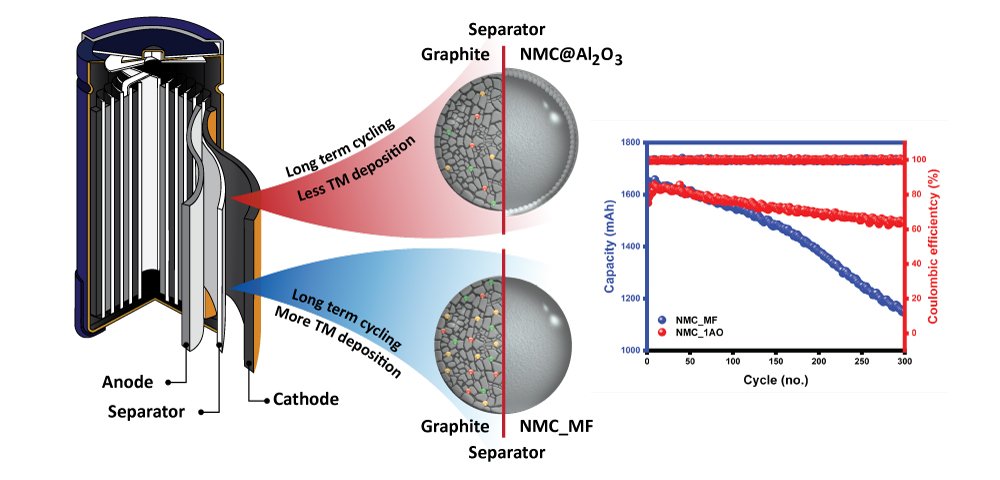

8. Microcracking of Ni-rich Layered Oxide Does Not Occur at Single Crystal Primary Particles Even Abused at 4.7 V
Kan Homlamai, Nichakarn Anansuksawat, Nattanon Joraleechanchai, Poramane Chiochan, Thitiphum Sangsanit, Worapol Tejangkura, Thana Maihom, Jumras Limtrakul, Montree Sawangphruk
Chem. Commun, 2022, 58, 11382-11385
DOI: https://pubs.rsc.org/en/content/articlehtml/2022/cc/d2cc03977j
Abstract: There is a controversial issue based on the particle cracking of the Ni-rich layered oxide cathode materials whether it occurs at the primary particles or the grain boundary. Herein, we found that the microcracking of NMC811 does not occur at single crystalline primary particles even abused at a severe upper cell voltage of 4.7 V having a lot of gas evolution since the single-crystal NMC811 has superior mechanical stability. The capacity retentions determined at 1C rate and a 100% state of charge (SOC) are 80% and 50% after 1000 cycles for single crystal and polycrystal NMC811, respectively.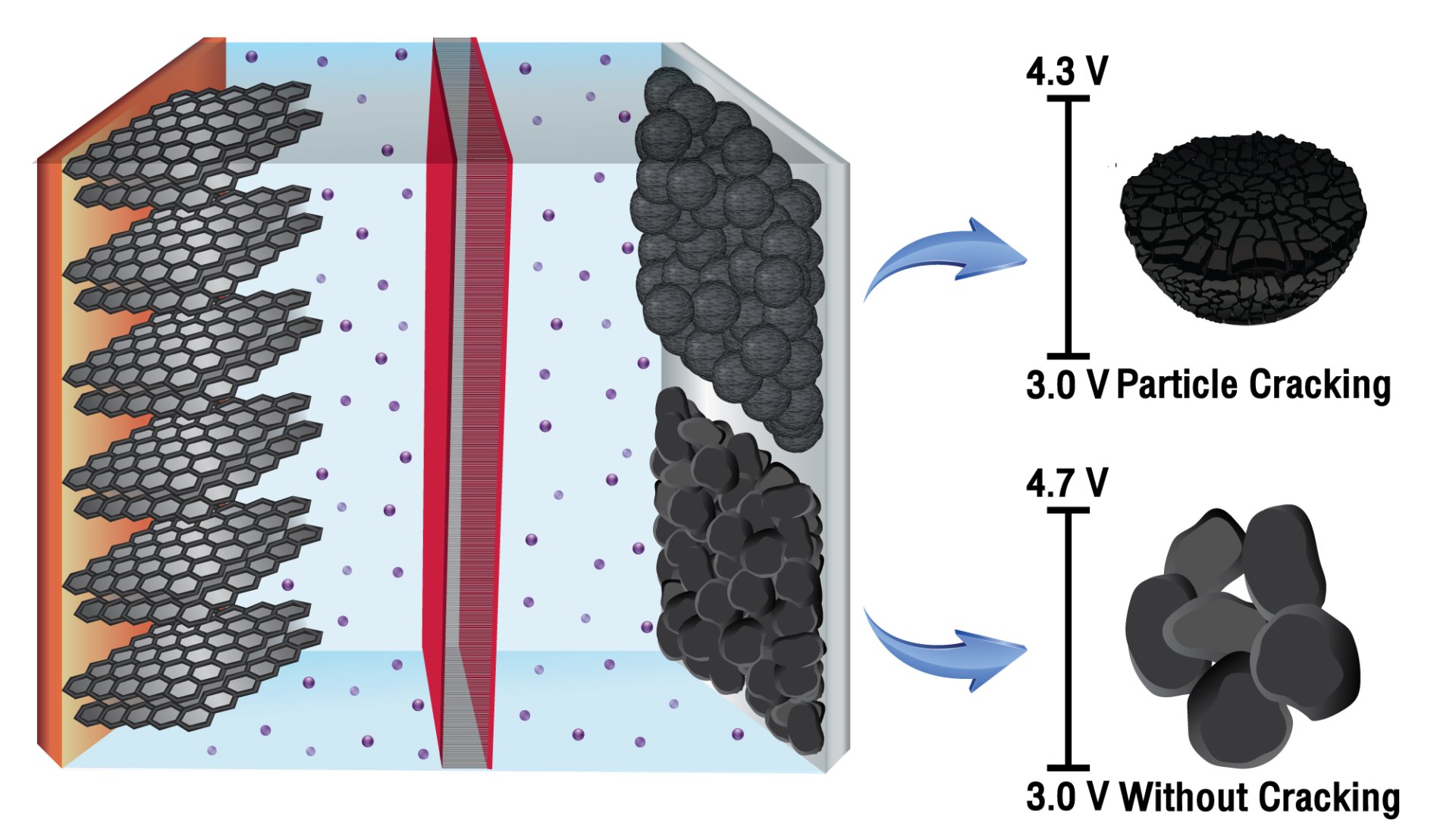

9. Mechanofusing Garnet Solid Electrolyte on the Surface of Ni-rich Layered Oxide Cathode towards High-Rate Capability of Cylindrical Li-ion Battery Cells
Panyawee Bunyanidhi, Nutthaphon Phattharasupakun, Chanikarn Tomon, Salatan Duangdangchote, Pinit Kidkhunthod, Montree Sawangphruk
Journal of Power Sources. 2022, 549, 232043
DOI: https://doi.org/10.1016/j.jpowsour.2022.232043
Abstract: The interface between solid electrolytes and cathode materials determines charge storage mechanism of Li-ion batteries; however, it has not yet been fully investigated and understood. Herein, a diluted amount at 1 wt% of heterogenous lithium garnet Li7La3Zr2O12 (LLZO) solid electrolyte particles was chemically bonded to the Ni-rich LiNi0.8Mn0.1Co0.1O2 (NMC811) cathode by a scalable mechanofusion process. The interface chemistry was intensively investigated by an ex situ extended X-ray absorption fine structure (EXAFS). In situ X-ray diffraction was also used to investigate the charge storage mechanism of LLZO-NMC811. It was found that the LLZO-LaNiO3-NMC811 strong bond at the interface can enhance the microstructural stability leading to high-rate capability up to 7.5C. For the charge storage mechanism, the lithium intercalation/deintercalation in NMC-LLZO undergoes a slower phase transition since the LLZO-LaNiO3 interface can modulate Li concentration gradient and kinetics where the surface of NMC will not be fully emptied (during charge) or filled (during discharge). This will benefit the rate capability of LLZO-modified NMC material in which the Li concentration at the surface will not quickly reach a threshold value before the cut-off voltage. The practical applications of LLZO-NMC811 were eventually demonstrated in 2.1 Ah 18650-format cells with high-capacity retention and high-rate capability.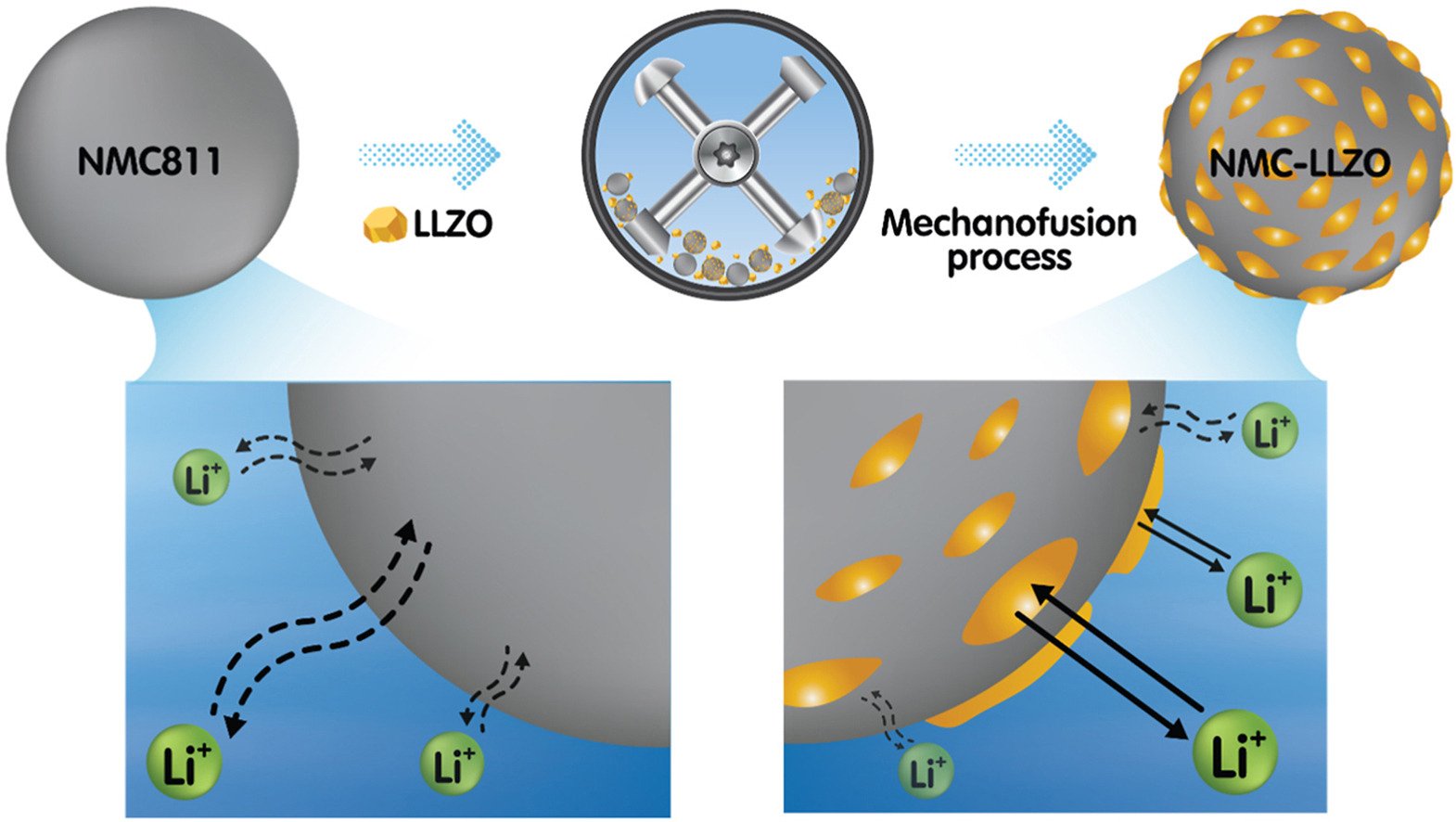

10. Effect of charging protocols on electrochemical performance and failure mechanism of commercial level Ni-rich NMC811 thick electrode
Nutthaphon Phattharasupakun, Panyawee Bunyanidhi, Poramane Chiochan, Narong Chanlek, Montree Sawangphruk
Electrochemistry Communications, 2022, 139, 107309
DOI: https://doi.org/10.1016/j.elecom.2022.107309
Abstract: In order to bridge the gap between academia and industry in the development of cathode active material for high-energy lithium-ion batteries (LIBs), several experimental factors reflecting commercial use need to be considered. Herein, based on a thick commercial level NMC811 electrode, we simply demonstrated the effect of charging protocols on the rate performance, cycling stability, and Li storage mechanism. A constant current charge/discharge, typically used in most publications, was found to exhibit worse rate capability and cycling stability compared to the constant current constant voltage (CCCV) mode of charge, used in commercial LIB cells due to the less uniform Li concentration gradient between the surface and bulk of the material. In operando XRD also revealed that there was no negative impact from the rapid change in lattice parameters towards cycling performance. We also found that the major degradation mechanism of Ni-rich NMC811 showing poor cycling performance across literature, can be mainly ascribed to the thick cathode electrolyte interphase (CEI) resistive layer, hindering Li intercalation at high rate and also lead to lower accessible capacity in each cycle. This work illustrates a simple example of the gaps between research in academic and industry that need to be narrowed for the development of practical high energy materials.
11. Atomic Force Microscopy: An Advanced Imaging Technique—From Molecules to Morphologies
Jeevan Kumar Reddy Modigunta, Selvamani Vadivel, G. Murali, Insik In, Montree Sawangphruk
Microscopic Techniques for the Non-Expert, 2022
DOI: https://link.springer.com/chapter/10.1007/978-3-030-99542-3_5
Abstract: Atomic force microscopy (AFM) is an extensively used advanced characterization technique for a nanoscale range of materials. This chapter clearly describes the importance and advantages of AFM, its working principles, modes of measurement, and its applications in interdisciplinary fields such as chemistry, materials science, and biology.
12. Diffusion of Zirconium (IV) Ions from Coated Thick Zirconium Oxide Shell to the Bulk Structure of Ni-rich NMC811 Cathode Leading to High-performance 18650 Cylindrical Li-ion Batteries
Suchakree Tubtimkuna, Nutthaphon Phattharasupakun, Panyawee Bunyanidhi, Montree Sawangphruk
Advanced Materials Technology, 2022
DOI: https://doi.org/10.1002/admt.202200436
Abstract: Herein, Ni-rich LiNi0.8Mn0.1Co0.1O2 or NMC811 cathode material, which is expected to be widely used soon, is coated by crystalline ZrO2 nanoparticles using green and scalable mechanofusion technique with an annealing process. A controllable synergistic effect of ZrO2 coating, as a spherical core–shell morphology with low surface energy, which is ideal for the process of electrode fabrication, and Zr4+ doping is carefully investigated. For the first time, the mechanofusion with the post-annealing at 800 °C used in this work can finely tune the shell thickness and doping gradient by the diffusion of Zr4+ from the coated ZrO2 shell to the bulk structure of NMC811. The optimized material, namely NMC@Zr-800 used as the cathode of 18650 cylindrical Li-ion batteries (LIBs), can provide excellent capacity retention over 1000 cycles at a severe 100% state-of-charge (SOC) at 1.0 C. Postmortem analysis shows that the material is stable with less crack formation and transition metal (TM) dissolution than the pristine NMC811 material owing to a synergistic effect of the surface protection by ZrO2 coating and Zr4+ doping. The results demonstrate the practical and scalable approach that will be beneficial for technological advancement in the high-energy 18650 cylindrical LIBs.
13. Regulating the Cationic Rearrangement of Ni-rich Layered Oxide Cathode for High-performance Li-ion Batteries
Selvamani Vadivel, Krisara Srimanon, Montree Sawangphruk
Journal of Power Sources, 2022, 537, 231526
DOI: https://www.sciencedirect.com/science/article/pii/S037877532200533X?dgcid=author
Abstract: Enormous effort has been paid to improve Ni-rich cathode's performance by suppressing surface residues and enhancing microscopic surface ordering (high Ni3+/Ni2+). Herein, strong alkali-mediated chemical oxidation of commercial precursor is employed to directly synthesize Ni-rich NCA cathode sintering under an oxygen atmosphere. Because of the limited lithium source, the lithium residue over the polycrystalline material is controlled; of course, a certain fraction of lithium has been lost and substituted by Ni2+, even pre-oxidized the precursor. XPS studies suggest that the percentage of oxidized nickel (Ni3+) is comparably higher at the surface than core (∼100 nm in depth) and retained during the lithiation. The synthesized material initially shows a high reversible lithiation efficiency of 90.6% at 0.05 C. The capacity degradation in the life-cycle study at 0.2 C could be endorsed to the combination of lithium inventory and active metal loss along with typical kinetic limitations, as diagnosed from the derivative dQ/dV plot. The high initial coulombic efficiency and discharge capacity with low polarization further confirm superior surface ordering and low surface residue. This study demonstrates that the pretreatment of the precursor is one of the effective strategies to regulate the cationic arrangement to achieve improved electrochemical performance.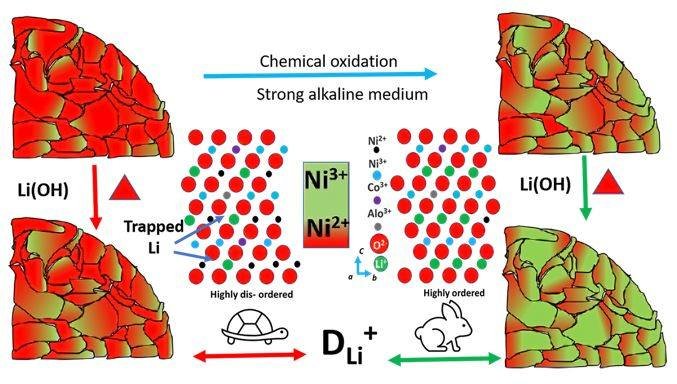

14. Core-shell structure of LiMn2O4 cathode material reduces phase transition and Mn dissolution in Li-ion batteries
Chanikarn Tomon, Sangchai Sarawutanukul, Nutthaphon Phattharasupakun, Salatan Duangdangchote, Praeploy Chomkhuntod, Nattanon Joraleechanchai, Panyawee Bunyanidhi, Montree Sawangphruk
Communications Chemistry, 2022, 5 , 54
DOI: https://www.nature.com/articles/s42004-022-00670-y
Abstract: Although the LiMn2O4 cathode can provide high nominal cell voltage, high thermal stability, low toxicity, and good safety in Li-ion batteries, it still suffers from capacity fading caused by the combination of structural transformation and transition metal dissolution. Herein, a carbon-coated LiMn2O4 cathode with core@shell structure (LMO@C) was therefore produced using a mechanofusion method. The LMO@C exhibits higher cycling stability as compared to the pristine LiMn2O4 (P-LMO) due to its high conductivity reducing impedance growth and phase transition. The carbon shell can reduce direct contact between the electrolyte and the cathode reducing side reactions and Mn dissolution. Thus, the cylindrical cell of LMO@C//graphite provides higher capacity retention after 900 cycles at 1 C. The amount of dissoluted Mn for the LMO@C is almost 2 times lower than that of the P-LMO after 200 cycles. Moreover, the LMO@C shows smaller change in lattice parameter or phase transition than P-LMO, indicating to the suppression of λ-MnO2 phase from the mixed phase of Li1-δMn2O4 + λ-MnO2 when Li-delithiation at highly charged state leading to an improved cycling reversibility. This work provides both fundamental understanding and manufacturing scale demonstration for practical 18650 Li-ion batteries.
15. The charge density of intercalants inside layered birnessite manganese oxide nanosheets determining Zn-ion storage capability towards rechargeable Zn-ion batteries
Praeploy Chomkhuntod, Kanit Hantanasirisakul, Salatan Duangdangchote, Nutthaphon Phattharasupakuna, Montree Sawangphruk
Journal of Materials Chemistry A, 2022, 10, 5561-5568
DOI: https://pubs.rsc.org/en/content/articlelanding/2022/ta/d1ta09968j
Abstract: Rechargeable aqueous Zn–MnO2 batteries have been considered as one of the promising alternative energy technologies due to their high abundance, environmental friendliness, and safety of both Zn–metal anodes and manganese oxide cathodes. Although layer-type MnO2 (δ-MnO2) is one of the most promising intercalation cathode materials, there are some critical drawbacks such as sluggish Zn2+ diffusion kinetics and a phase transition of δ-MnO2 as a result of a strong electrostatic interaction between Zn2+ and the host structure. Herein, we systematically studied the effects of the charge density of pre-intercalated cations in layered MnO2 using Li+, Ca2+, and Al3+ on its structural properties and electrochemical performance as a cathode in aqueous Zn–MnO2 batteries. The results reveal that a small amount of highly charged intercalant can effectively stabilize the MnO2 layers, facilitating the kinetics of Zn2+ intercalation/deintercalation. As a result, Al–MnO2 exhibits superior capacity and a rate capability of 210 mA h g−1 with 21% capacity retention when the current density is increased from 0.1 to 2 A g−1, while Ca–MnO2 and Li–MnO2 exhibit 189 and 160 mA h g−1 with a capacity retention of 17% and 11%, respectively. The superior capacity of Al–MnO2 is attributed to the enhanced redox activity from more Mn electrochemical utilization as confirmed by ex situ X-ray photoelectron spectroscopy. Moreover, the long-term cycling stability evaluated at 2 A g−1 shows that Al–MnO2 exhibits superior cycling stability with 84% capacity retention over 2000 cycles. As revealed by ex situ X-ray diffraction and theoretical calculations, the highly charged intercalant can minimize the binding energy between Zn2+ and the MnO2 host, alleviating the strong electrostatic attraction that can induce reversible Zn2+ insertion/extraction.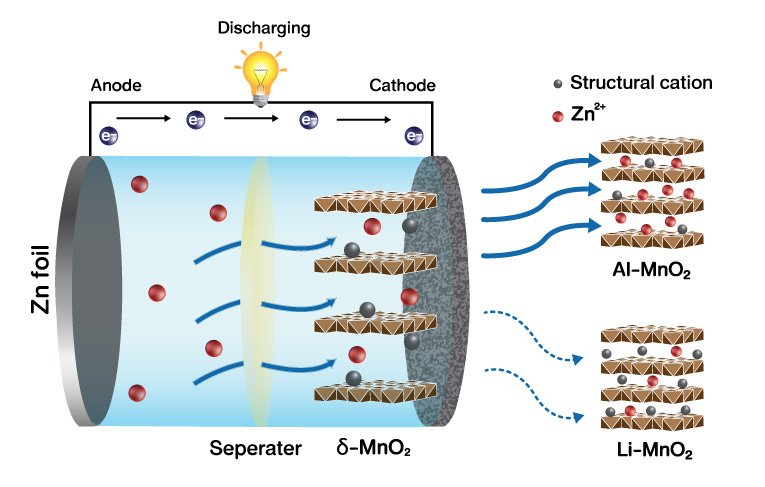

16. Enzyme-immobilized 3D silver nanoparticle/graphene aerogel composites towards biosensors
Wongduan Sroysee, Ketsuda Kongsawatvoragul, Phitchayapha Phattharaphuti, Pattranit Kullawattanapokin, Chonticha Jangsan, Worapol Tejangkura, Montree Sawangphruk
Materials Chemistry and Physics, 2022, 277, 125572
DOI: https://doi.org/10.1016/j.matchemphys.2021.125572
Abstract: The detection of allergen sulfite is essential for food quality control and public health supervision. Here, a high-performance sulfite biosensor was developed from a sulfite oxidase enzyme (SOx) immobilized on silver nanoparticles (AgNPs) decorated on 3D reduced graphene oxide (3D-rGO). 3D-rGO with high specific pore volume and high electroactive surface area is ideal as a supporting material. AgNPs further provide high electrical conductivity or fast charge transfer and serve as an enzyme anchoring site via a stable thiol bonding. The composite material was initially functionalized with the folic acid and cysteine namely rGO@Ag-Cys-FA before immobilized with the SOx. The resultant rGO@Ag-Cys-FA-SOx exhibits high sensitivity and selectivity towards sulfite detection, high bio-electrocatalytic activity, and fast heterogeneous electron transfer rate constant. The biosensor is also tested in a continuous flow injection system to demonstrate the practical use. Besides, the as-produced sensor in this work can be used in the real sample, which is the canned fruit product indicating its potential practical application.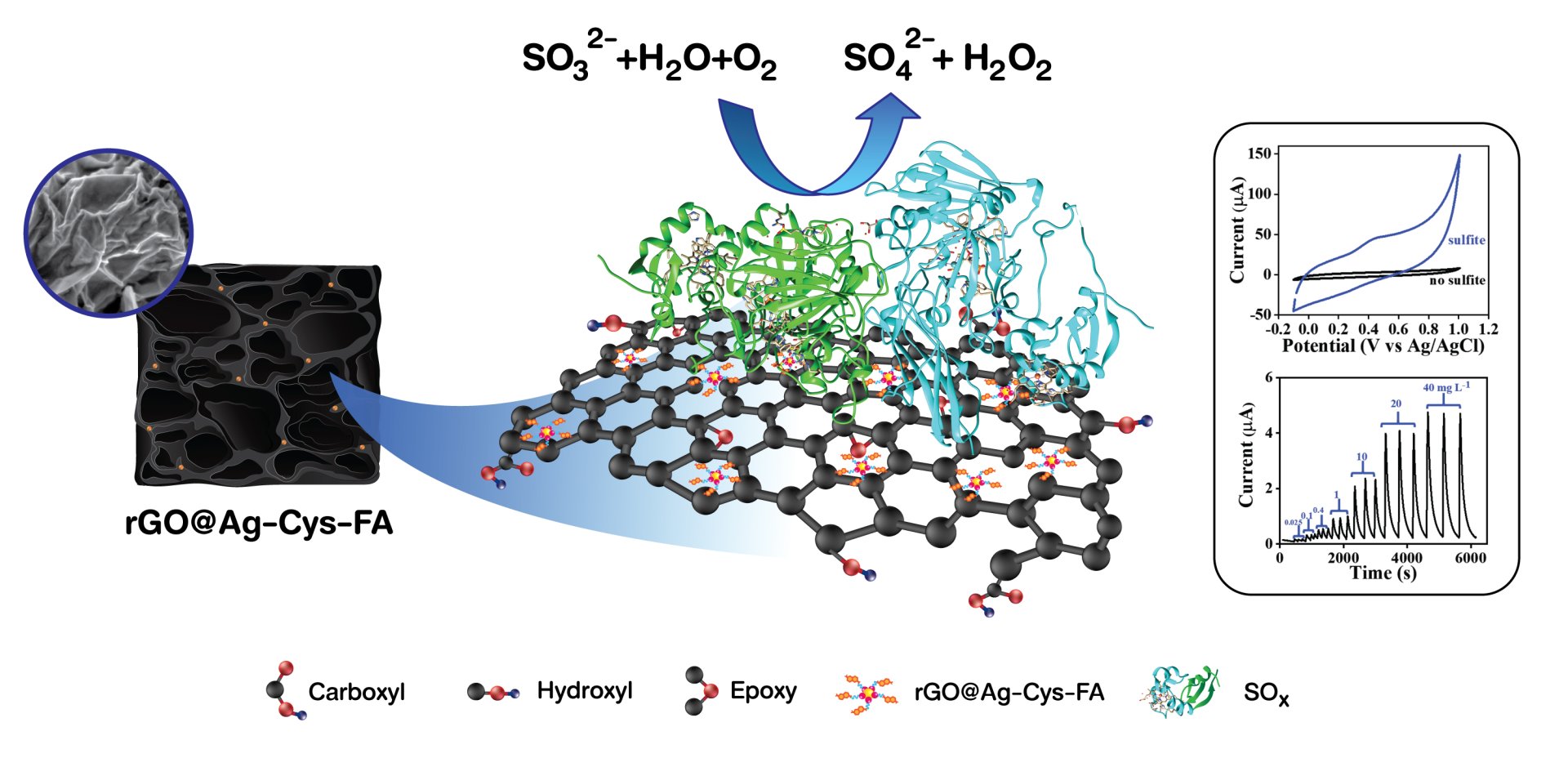

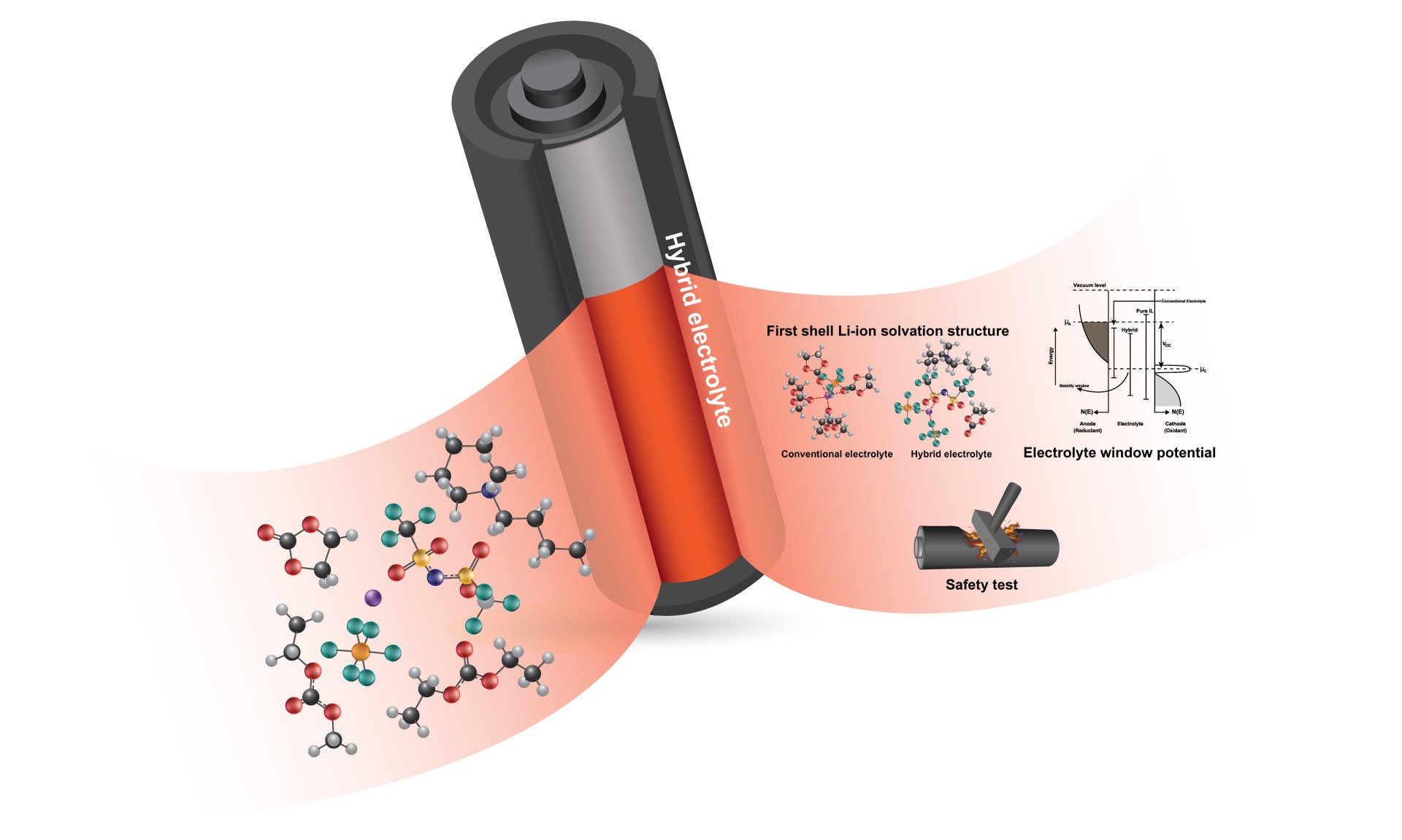
17. Free carbonate-based molecules in the electrolyte leading to severe safety concern of Ni-rich Li-ion batteries
Nattanon Joraleechanchai, Ruttiyakorn Donthongkwa, Salatan Duangdangchote, Nutthaphon Phattharasupakun, Poramane Chiochan, Kan Homlamai and Montree Sawangphruk
Chemical Communications, 2022, 58, 779-782
DOI: https://doi.org/10.1039/D1CC06694C
Abstract: The safety of Li-ion batteries is one of the most important factors, if not the most, determining their practical applications. We have found that free carbonate-based solvent molecules in the hybrid electrolyte system can cause a severe safety concern. Mixing ionic liquids to the carbonate-based solvent as the co-solvent at fixed 1M LiPF6 salt can lead to free carbonate-based molecules causing poor charge storage performance and safety concerns.