1. Rational design and synthesis of SiC/TiC@SiOx/TiO2 porous core-shell nanostructure with excellent Li-ion storage performance
By: Li, Chenyang; Qin, Jiaqian; Sawangphruk, Montree; et al.
Chemical Communications, 2018, 54, 12622-12625
DOI : https://pubs.rsc.org/en/content/articlelanding/2018/CC/C8CC07673A#!divAbstract
Abstract: Rational design and synthesis of a new silicon carbide/titanium carbide@silicon oxide/titanium dioxide (SiC/TiC@SiOx/TiO2) porous core–shell nanostructured material could significantly enhance the specific capacity, rate capability, and cycle stability of electrodes. The results demonstrate that the specific capacities are 387, 300, 252, 210, 174 and 151 mA h g−1 at 100, 200, 500, 1000, 1500 and 2000 mA g−1, respectively. The newly designed electrode still exhibits a larger specific capacity of 217 mA h g−1 at 1000 mA g−1 after the 1000th cycle.
2. Designing an interlayer of reduced graphene oxide aerogel and nitrogen-rich graphitic carbon nitride by a layer-by-layer coating for high-performance lithium sulfur batteries
By: Wutthiprom, Juthaporn; Phattharasupakun, Nutthaphon; Sawangphruk, Montree
CARBON, 2018, 139, 945-953
DOI : https://www.sciencedirect.com/science/article/pii/S0008622318307346?via%3Dihub
Abstract: Herein, reduced graphene oxide aerogel (GA) with 3D interconnected conductive and porous structure was used as a host of sulfur (S@GA cathode). GA was also used together with nitrogen-rich graphitic carbon nitride (GCN) as the interlayer accommodating the soluble lithium polysulfides (LPSs). The interlayer was fabricated by a layer-by-layer coating technique on the flexible and processible carbon fiber paper (CFP) substrate. The interlayer inserted between the cathode and the separator provides a strong lithium polysulfide adsorptivity. Adsorptive LPS species on the interlayer were also investigated by an ex situ X-ray photoelectron spectroscopy. After finely tuned, the as-fabricated LSB using the S@GA cathode with the GA/GCN interlayer exhibits 75% higher specific capacity than the cell without interlayer. It has a low capacity fading of 0.056% per cycle after tested for 800 cycles. Our new design and configuration of LSB may practically be used in high-energy applications since it can effectively address many issues of the current LSBs.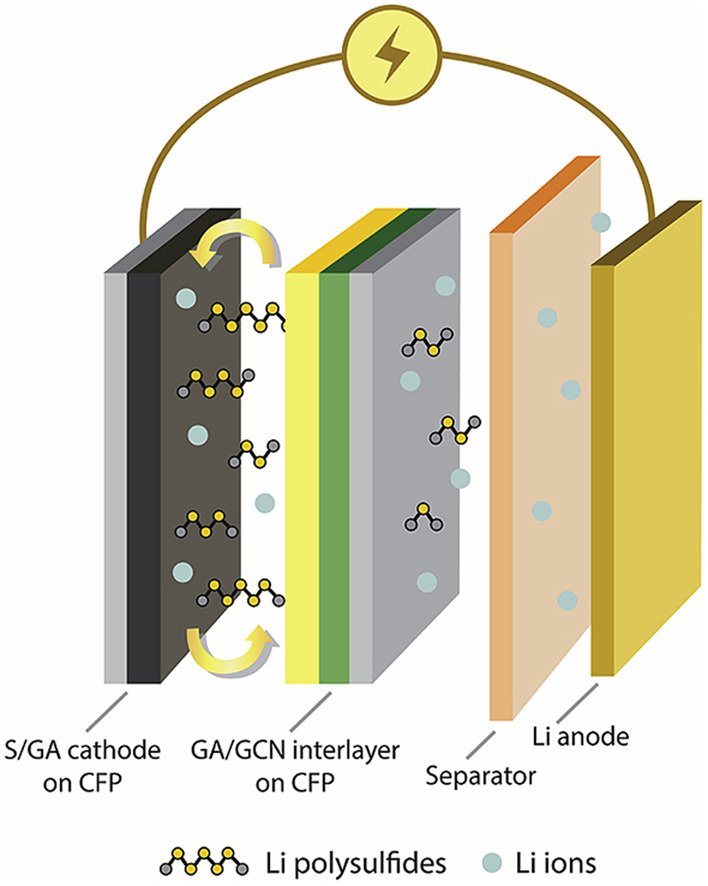

3. Facile Electrodeposition of Ni-Cu-P Dendrite Nanotube Films with Enhanced Hydrogen Evolution Reaction Activity and Durability
By: Cao, Meng; Xue, Zhe; Niu, Jingjing; et al.
ACS APPLIED MATERIALS & INTERFACES, 2018, 10, 35224-35233
DOI : https://pubs.acs.org/doi/full/10.1021/acsami.8b12321
Abstract: Hydrogen can be the potential substitute energy carrier for fuel while electrolysis water with hydrogen evolution reaction (HER) is an efficient way to produce hydrogen. Highly active and robust electrocatalysts composed by earth abundant elements are required. Herein, nickel–copper–phosphorus (Ni–Cu–P) electrocatalysts are designed and synthesized by a facile one-step electrodeposition method. A unique pine-needle-like dendrite nanotube morphology of Ni–Cu–P electrocatalyst can be synthesized when copper content changed and impressive HER activity obtained in alkaline and acidic media. Briefly, the overpotential reaches 120 mV in 1 M KOH and 150 mV in 0.5 M H2SO4 at the current density of 10 mA cm–2, with the corresponding Tafel slope reaching 69 mV dec–1. The results are close to that of commercial Pt/C catalysts (37 mV in 1 M KOH). Furthermore, the density functional theory calculations also demonstrate that P-incorporated Ni–Cu, Cu-incorporated Ni–P, and Ni-incorporated Cu–P have the optimized hydrogen adsorption free energy (ΔGH*) of −0.066, −0.157, and −0.003 eV, respectively, which are more suitable than those of Ni–Cu, Ni–P, and Cu–P, respectively. The Ni-incorporated Cu–P even has a much smaller ΔGH* of −0.003 than that of Pt (∼−0.09 eV). We believe that our study will provide a new strategy to design non-noble metal alloy materials for practical applications in catalysis and energy fields.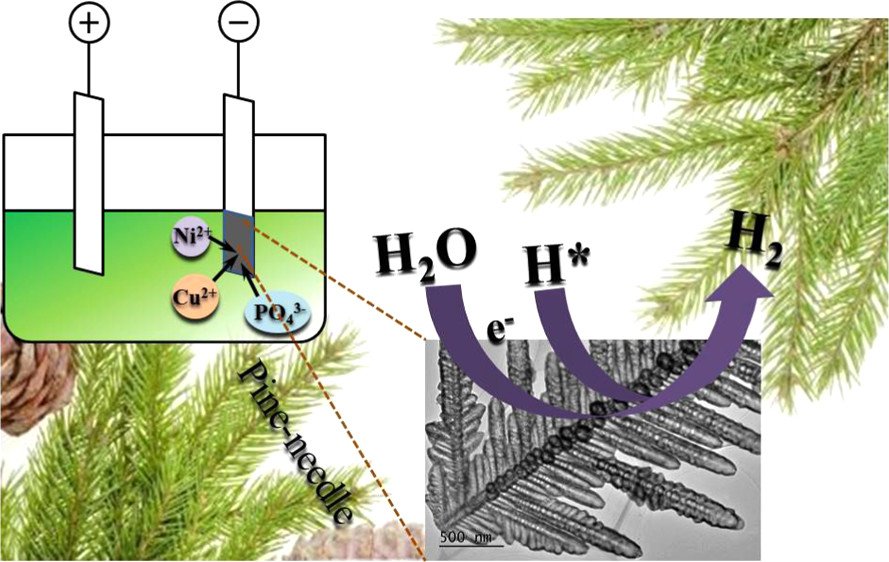

4. Electrolyte-Induced Electrical Disconnection between Single Graphene Nanoplatelets and an Electrode
By: Krittayavathananon, Atiweena; Li, Xiuting; Batchelor-McAuley, Christopher; et al.
JOURNAL OF PHYSICAL CHEMISTRY LETTERS, 2018, 9, 5822–5826
DOI : https://pubs.acs.org/doi/10.1021/acs.jpclett.8b02296
Abstract: We report the influence of electrolyte on the electrical contact between graphene nanoplatelets (GNPs) and an electrode via a single entity electrochemical technique. The current “steps” were observed in the absence of electrolyte due to the GNPs impacting on and bridging across an interdigitated gold electrode array (IDE); in contrast, current spikes of short duration were obtained in the presence of electrolyte. This result indicates that in the latter case the constant short-circuit bridging current was switched off and replaced solely by impacts of GNPs with single electrodes. These observed current spikes measured in the presence of electrolyte are evidenced to be of a capacitative nature, demonstrating the high sensitivity of the electronic properties of the GNP/metal junction to the ionic strength of the electrolytic solution.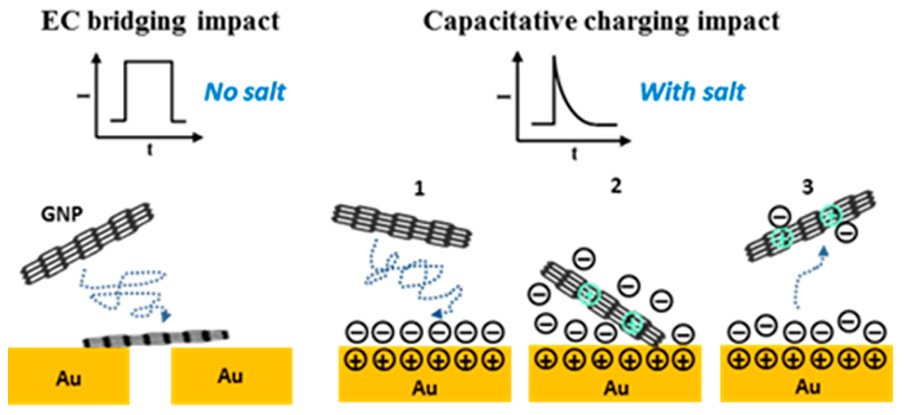

5. Sodium-ion diffusion and charge transfer kinetics of sodium-ion hybrid capacitors using bio-derived hierarchical porous carbon
By: Phattharasupakun, Nutthaphon; Wutthiprom, Juthaporn; Ma, Nattapol; et al.
Electrochimica Acta, 2018 ,286, 55-64
DOI : https://www.sciencedirect.com/science/article/pii/S0013468618317961
Abstract: Even though Li-ion based energy storages can provide high specific energy and good cycling stability, the scarcity and high price of Li-based materials may limit their applications in the future. In this work, we have investigated Na-ion hybrid capacitors (NICs) with both high specific energy and power. Bio-derived or Jasmine rice-derived hierarchical porous carbon (j-HPC) with a specific BET surface area of 2377 m2 g−1 and a mean pore diameter of 2.53 nm containing 73.17 at.% C, 2.24 at.% N, and 24.59 at.% O is used as a new electrode of NICs. We have found that a fast Na ion diffusion of 10−8-10−11 cm2 s−1 and a fast-standard heterogeneous rate constant of electron transfer of ca. 10−5 cm s−1 are two reasons leading to high-performance NIC. The NIC exhibits a maximum operating cell voltage of 3.8 V, a maximum specific energy of 116 Wh kg−1 (142 μWh cm−2) and a maximum specific power of 11,121 W kg−1 (13,618 μW cm−2) with 90% capacity retention after 5000 cycles. Our NIC using j-HPC may be an ideal device for high power and energy storage technology.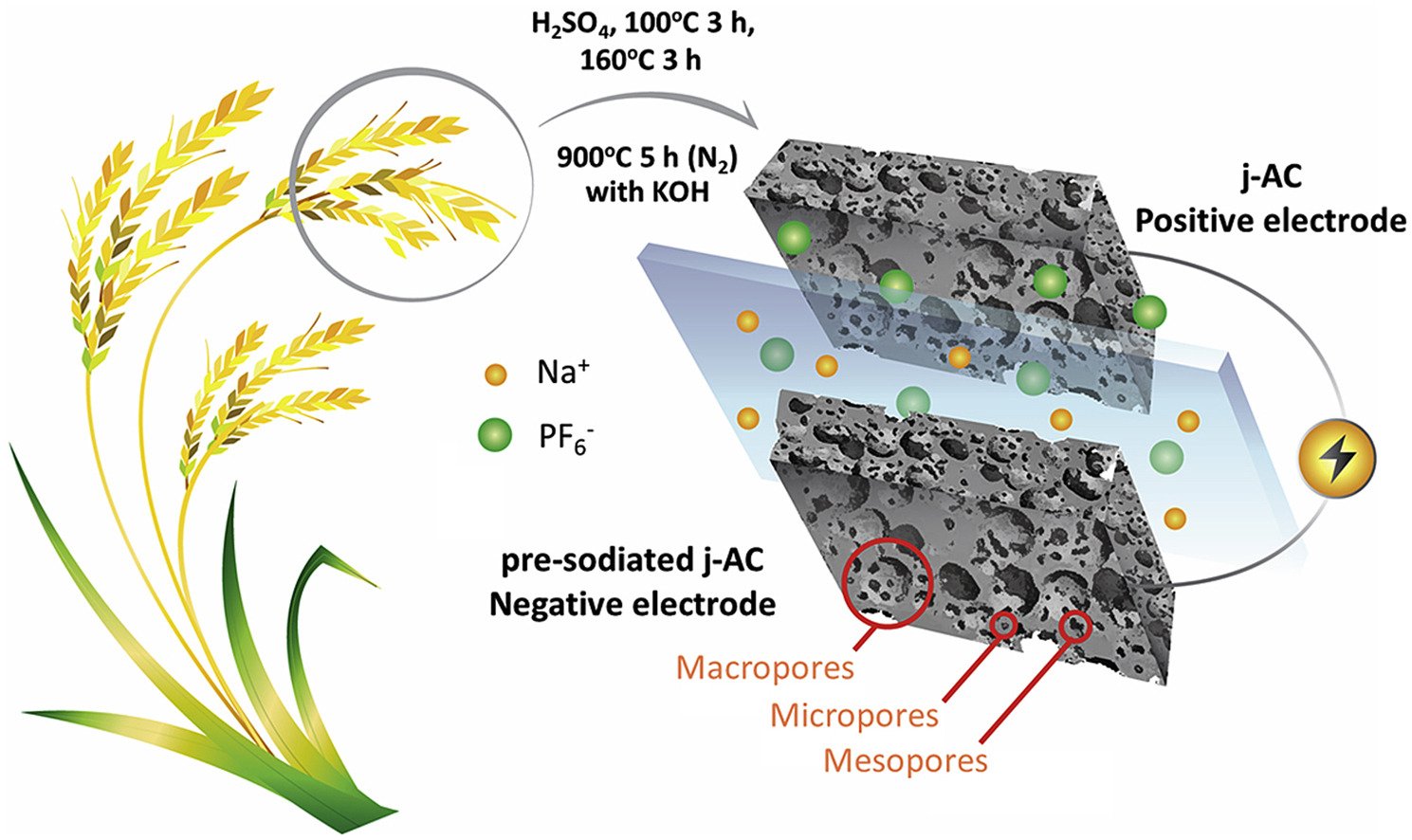

6. Environmentally benign non-fluoro deep eutectic solvent and free-standing rice husk-derived bio-carbon based high-temperature supercapacitors
By: Sathyamoorthi, Sethuraman; Phattharasupakun, Nutthaphon; Sawangphruk, Montree
Electrochimica Acta, 2018, 286, 48-157
DOI : https://www.sciencedirect.com/science/article/pii/S0013468618317948
Abstract: Deep eutectic solvents (DESs) have been emerging as newer environmentally benign electrolytes for energy storage devices with the high-temperature operational capability. For the first-time, we propose ethylene glycol and tetrapropylammonium bromide based non-fluoro DESs as electrolytes for the supercapacitor. Different compositions of DESs were also evaluated. Also, the high-temperature operational capability of the proposed electrolytes was evaluated with the free-standing microporous commercial activated carbon (AC) and mesoporous rice husk-derived bio-activated carbon (Bio-AC). The maximum cell voltages of 1.3 V and 1.0 V are identified for AC and Bio-AC based supercapacitors, respectively at 25 °C. The specific capacitances of both AC and Bio-AC electrodes are ∼100 F g−1 at 0.1 A g−1. The proposed DES electrolyte can be operated at the maximum temperature of 115 °C, which is higher than the previously reported DESs (80 °C). The specific capacitance increases with increasing temperature, and the maximum specific capacitance of an electrode is 172 F g−1 at 115 °C for Bio-AC supercapacitor. Excellent long-term stability is noted after 10,000 cycles at 25 °C and 115 °C for both AC and Bio-AC supercapacitors. The supercapacitors here may be practically used in high-temperature applications.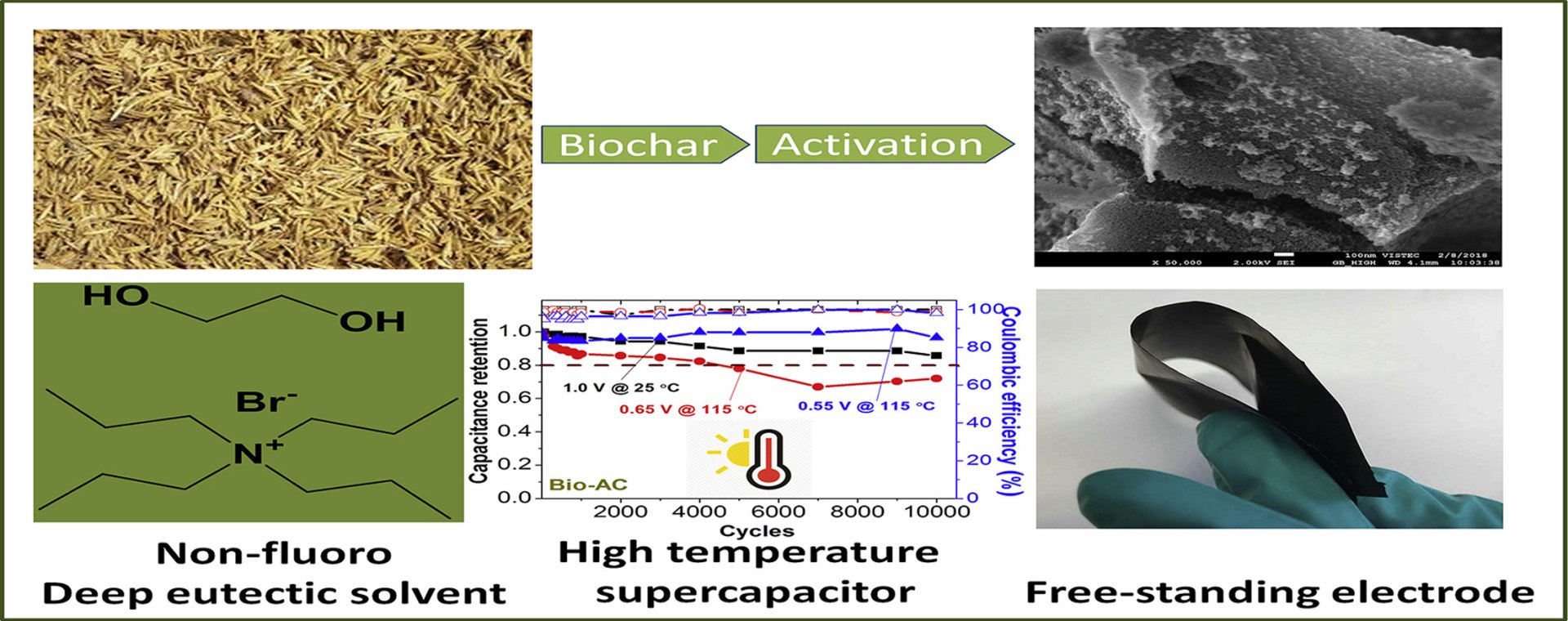

7. Asymmetric hybrid energy conversion and storage cell of thin Co2O4 and N-doped reduced graphene oxide aerogel films
By: Kalasina, Saran; Phattharasupakun, Nutthaphon; Suksomboon, Montakan; et al.
Electrochimica Acta, 2018, 283, 1125-1133
DOI : https://www.sciencedirect.com/science/article/pii/S0013468618315482
Abstract: Although batteries and supercapacitors can store electrical energy, they cannot convert energy like solar cells. Here, we introduce a new asymmetric hybrid energy conversion and storage (HECS) cell consisting of thin photoactive cobalt oxide (Co3O4) film with a thickness of 1 μm coated on the fluorine-doped tin oxide (FTO) glass as the positive electrode and thin N-doped reduced graphene oxide aerogel (N-rGOAE) film with a thickness of 10 μm coated on the FTO glass as the negative electrode. The HECS cell can convert light to electricity, which is subsequently stored within its electrode materials. The as-fabricated asymmetric HECS cell of Co3O4//N-rGOAE thin films exhibits 140% higher specific capacitance under red LED light illumination than that under dark condition and provides a specific energy and a specific power of ca. 3.5 Wh kg−1 and ca. 7000 W kg−1, respectively. The Co3O4 can absorb all visible light spectrum and excite its valence electron to the conduction band via its indirect band gap (1.45 eV) so-called the photovoltaic effect. Whilst, the N-rGOAE can balance all charges generated at the Co3O4 positive electrode via the electrochemical double-layer capacitance. The asymmetric HECS of Co3O4//N-rGOAE may be an ideal solution for future renewable and sustainable energy.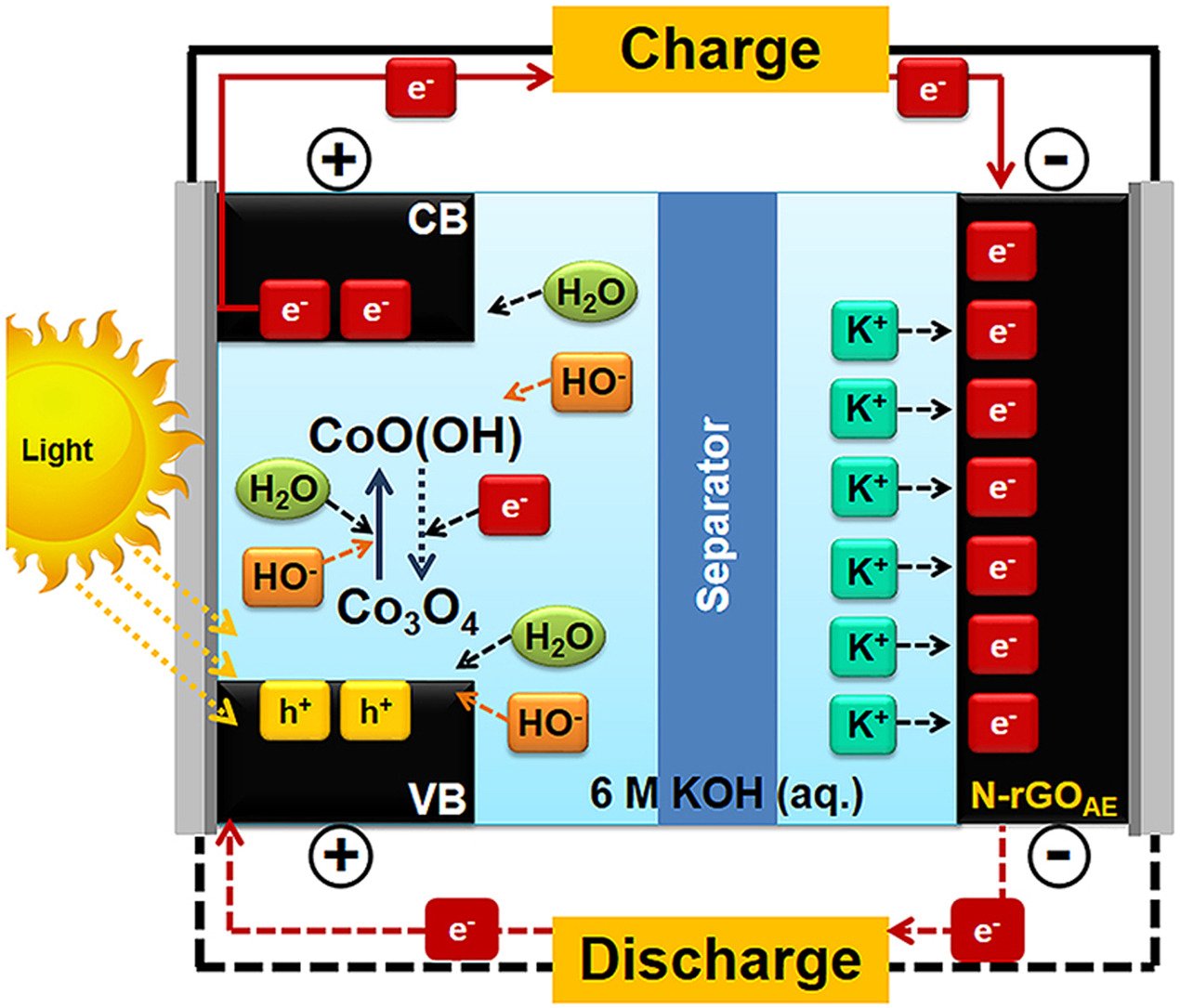

8. Comparing the effect of different surfactants on the aggregation and electrical contact properties of graphene nanoplatelets
By: Krittayayathananon, Atiweena; Li, Xiuting; Batchelor-McAuley, Christopher; et al.
Applied Materials Today, 2018, 12, 163-167
DOI : https://www.sciencedirect.com/science/article/pii/S2352940718301495?via%3Dihub
Abstract: We report the influence of different surfactants, Triton X-100, sodium dodecyl sulfate (SDS) and cetrimonium bromide (CTAB), on the suspended graphene nanoplatelets (GNPs) in aqueous solution studied via a single entity electrochemical technique. This method simply requires recording the current responses resulting from the impacts of individual and aggregated GNPs with an interdigitated gold electrode array. Upon impact the nanomaterial can bridge across two interdigitated gold microbands creating an electrical contact. The current magnitude reflects both the agglomeration state of the material and the contact resistance between the gold array and the carbon nanomaterial. The sensitivity of the magnitude of the current steps to the identity of the used surfactant was further evidenced, indicating the influence of these surfactants upon the electrical connectivity of the nanomaterial to the electrode substrate.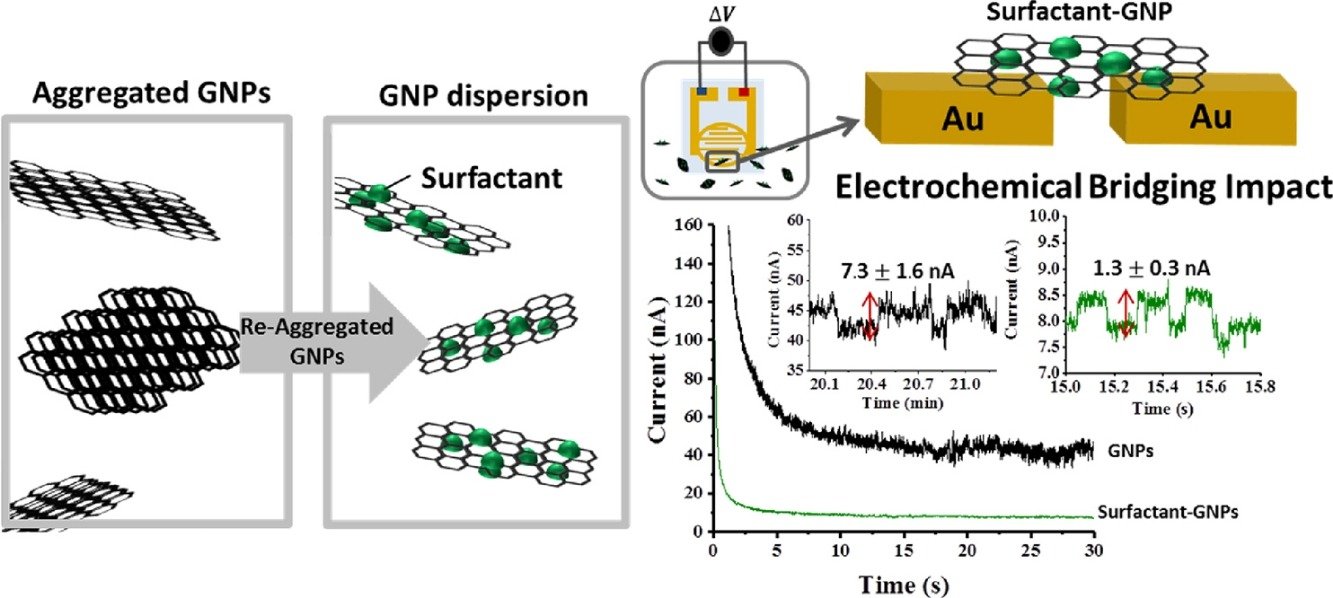

9. Novel Hybrid Energy Conversion and Storage Cell with Photovoltaic and Supercapacitor Effects in Ionic Liquid Electrolyte
By: Kalasina, Saran; Phattharasupakun, Nutthaphon; Maihom, Thana; et al.
Scientific Reports, 2018, 8, 12192
DOI : https://www.nature.com/articles/s41598-018-30707-z
Abstract: A single hybrid energy conversion and storage (HECS) cell of alpha-cobalt hydroxide (α-Co(OH)2) in ionic liquid was fabricated and operated under light illumination. The α-Co(OH)2, which is unstable in an aqueous electrolyte (i.e. KOH), is surprisingly stable in 1-butyl-1-methyl-pyrrolidinium dicyanamide ionic liquid. The as-fabricated HECS cell provides 100% coulombic efficiency and 99.99% capacity retention over 2000 cycles. Under a photo-charging condition, the dicyanamide anion of ionic liquid can react with a generated α-Co(OH)2+ hole at the positive electrode since the HOMO energy level of the anion is close to the valence band of α-Co(OH)2. The excited photoelectron will transfer to the current collector and move to the negative electrode. At the negative electrode, the 1-butyl-1-methyl-pyrrolidinium cations of ionic liquid do electrostatically adsorb on the surface and intercalate into the interlayer of active material stabilizing the whole cell. The HECS cell having both energy conversion (photovoltaic effect) and energy storage (supercapacitor) properties may be an ideal device for future renewable energy.
10. Insight into the effect of intercalated alkaline cations of layered manganese oxides on the oxygen reduction reaction and oxygen evolution reaction
Chemical Communications, 2018, 54, 8575-8578
DOI : https://pubs.rsc.org/en/content/articlelanding/2018/CC/C8CC03775B#!divAbstract
Abstract: The effect of the intercalated alkaline cations between the adjacent layers of multilayered manganese oxide (MnOx) towards the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) was investigated. Li–MnOx, Na–MnOx, K–MnOx, Rb–MnOx, and Cs–MnOx provide OER overpotentials of 1.64, 1.70, 1.79, 1.83, and 1.84 V vs. RHE, respectively as well as ORR overpotentials of 0.71, 1.06, 1.13, 1.15, and 1.14 V vs. RHE, respectively. Li–MnOx shows the highest bifunctional catalytic activity towards both the ORR and OER. In addition, the Gibbs free energy change of *OH adsorption is found to be the largest throughout the reaction pathways determining the rate of the whole ORR and OER.

11. Transparent supercapacitors of 2 nm ruthenium oxide nanoparticles decorated on a 3D nitrogen-doped graphene aerogel
By: Suktha, Phansiri; Phattharasupakun, Nutthaphon; Sawangphruk, Montree
Sustainable Energy & Fuels, 2018, 2, 1799-1805
DOI : https://pubs.rsc.org/en/content/articlelanding/2018/SE/C8SE00177D#!divAbstract
Abstract: Although ruthenium oxide nanoparticles (RuO2), graphene, and their composites have been widely used as supercapacitor electrode materials, transparent supercapacitors of these materials have been rarely investigated. In this work, we fabricated high-performance transparent solid-state supercapacitors of 2 nm RuO2 decorated on a 3D nitrogen-doped reduced graphene oxide aerogel (NGA) with 27–54% transparency. The as-fabricated symmetric supercapacitor of RuO2/NGA at a finely tuned mass loading of 16.3 μg cm−2 with a finely tuned transmittance of 34.1% at a wavelength of 550 nm exhibits a maximum areal energy of 0.074 μW h cm−2 and a maximum areal power of 64 μW cm−2. The cycling stability of the device can also be maintained at 100% over 2000 cycles. The high transparent supercapacitor in this work may practically be used in many advanced transparent electrical devices.
12. Bifunctional electrocatalytic CoNi-doped manganese oxide produced from microdumbbell manganese carbonate towards oxygen reduction and oxygen evolution reactions
By: Gatemala, Harnchana; Kosasang, Soracha; Sawangphruk, Montree
Sustainable Energy & Fuels, 2018, 2, 1170-1177
DOI : https://pubs.rsc.org/en/content/articlelanding/2018/SE/C8SE00062J#!divAbstract
Abstract: Herein, a new, simple, and stabilizer-free chemical synthetic protocol of microdumbbell manganese carbonate used as a precursor for producing nanoporous metal-doped manganese oxides is reported. This is the first time that a new growth mechanism of manganese carbonate via an oriented attachment mechanism of crystalline manganese carbonate nanospheres using its ferromagnetic property is revealed. The co-precipitation of divalent metal ions can be employed for the synthesis of metal-doped manganese carbonates. As-synthesized metal carbonates were employed as templates for producing porous metal-doped manganese oxides by calcination at 500–900 °C. The retention of template morphologies and metal oxide phases can be controlled by varying the calcination temperature. Doping of manganese oxide by Co2+ and Ni2+ to form a trimetal oxide can improve the catalytic performances for the ORR and OER under alkaline conditions. In addition, the trimetal oxide shows exceptional stabilities of 95.1% and 99.6% relative current retention for the ORR and OER at 40 000 s, respectively. The catalyst produced in this work may be practically used towards the ORR and OER in many systems such as metal–air batteries.
13. Oxidative chemical vapour deposition of a graphene oxide carbocatalyst on 3D nickel foam as a collaborative electrocatalyst towards the hydrogen evolution reaction in acidic electrolyte
By: Sarawutanukul, Sangchai; Phattharasupakun, Nutthaphon; Wutthiprom, Juthaporn; et al.
Sustainable Energy & Fuels, 2018, 2, 1305-1311
DOI : https://pubs.rsc.org/en/content/articlelanding/2018/SE/C8SE00161H#!divAbstract
Abstract: In this study, a graphene oxide (GO) carbocatalyst was synthesized as a thin film on a 3D Ni foam substrate (GO@Ni) by oxidative chemical vapour deposition (CVD) using methanol and water as precursors. The GO@Ni was used as a collaborative electrocatalyst towards the hydrogen evolution reaction (HER) in an acidic electrolyte. Notably, pure Ni metal cannot be used as an electrocatalyst in acidic media due to corrosion. The amount of water in the methanol was finely tuned to optimize the electrochemical activity of the GO@Ni for HER. The optimized GO@Ni catalyst, with a sheet resistivity of 133.48 Ω square−1, an optical band gap of 1.52 eV, and a C : O ratio of 3.89 produced using 90 : 10 V% of methanol : water, exhibits excellent and stable HER activity with an overpotential of 137 mV vs. RHE, reaching a high current density of 10 mA cm−2 and prominent electrochemical stability for up to 12 h in 0.5 M H2SO4. In addition, the CVD GO layer on the Ni foam acts as an anti-corrosion material protecting the Ni HER catalyst in the acidic environment. As a result, the GO@Ni may be a promising electrode for HER, replacing expensive Pt catalysts.
14. Fabrication and electrochemical properties of activated CNF/Cu (x) Mn1-x Fe2O4 composite nanostructures
By: Nilmoung, Sukanya; Sonsupap, Somchai; Sawangphruk, Montree; et al.
Applied Physics A Materials Science & Processing, 2018, 124, 427
DOI: https://link.springer.com/article/10.1007/s00339-018-1839-3
Abstract: This work reports the fabrication and electrochemical properties of activated carbon nanofibers composited with copper manganese ferrite (ACNF/Cu x Mn1−xFe2O4: x = 0.0, 0.2, 0.4, 0.6, 0.8) nanostructures. The obtained samples were characterized by means of X-ray diffraction, field emission scanning electron microscopy, Brunauer–Emmett–Teller analyzer, thermal gravimetric analysis, X-ray photoemission spectroscopy, and X-ray absorption spectroscopy. The supercapacitive behavior of the electrodes is tested using cyclic voltammetery, galvanostatic charge–discharge and electrochemical impedance spectroscopy. By varying ‘x’, the highest specific capacitance of 384 F/g at 2 mV/s using CV and 314 F/g at 2 A/g using GCD are obtained for the x = 0.2 electrode. The second one of 235 F/g at 2 mV/s using CV and 172 F/g at 2 A/g using GCD are observed for x = 0.8 electrode. The corresponding energy densities are 74 and 41 Wh/kg, respectively. It is observed that the cyclic stability of the prepared samples strongly depend on the amount of carbon, while the specific capacitance was enhanced by the sample with nearly proportional amount between carbon and CuMnFe2O4. Such results may arise from the synergetic effect between CuMnFe2O4 and ACNF.

15. Charge storage mechanisms of birnessite-type MnO2 nanosheets in Na2SO4 electrolytes with different pH values: In situ electrochemical X-ray absorption spectroscopy investigation
By: Tanggarnjanavalukul, Chan; Phattharasupakun, Nutthaphon; Wutthiprom, Juthaporn; et al.
Electrochimica Acta, 2018, 273, 17-25
DOI: https://www.sciencedirect.com/science/article/pii/S0013468618307564?via%3Dihub
Abstract: The charge storage mechanism of birnessite-type MnO2 nanosheet electrode with ca. 100 μm in thickness in 0.5 M Na2SO4 electrolytes with different pH values was investigated by an in situ electrochemical X-ray absorption spectroscopy (XAS) technique. It is found that the charge storage capacity of MnO2 is controlled by both surface adsorption and redox reaction. At pH 1 and 2, the rate of surface adsorption is faster than that of the surface redox reaction. Whilst, at pH 3, 4, and 5.9, the major capacitance originates from the redox reaction or the insertion/de-insertion of solvated cations into the layered MnO2 nanostructures. As a result, the MnO2-based supercapacitor electrode in 0.5 M Na2SO4 with diluted H2SO4 (pH 4) exhibits higher specific capacitance than that in electrolytes with pH 1–2. The decrease of capacitance at low pH is due to the partial dissolution of MnO2. This finding may lead to better understanding on the charge storage mechanism of the MnO2 materials.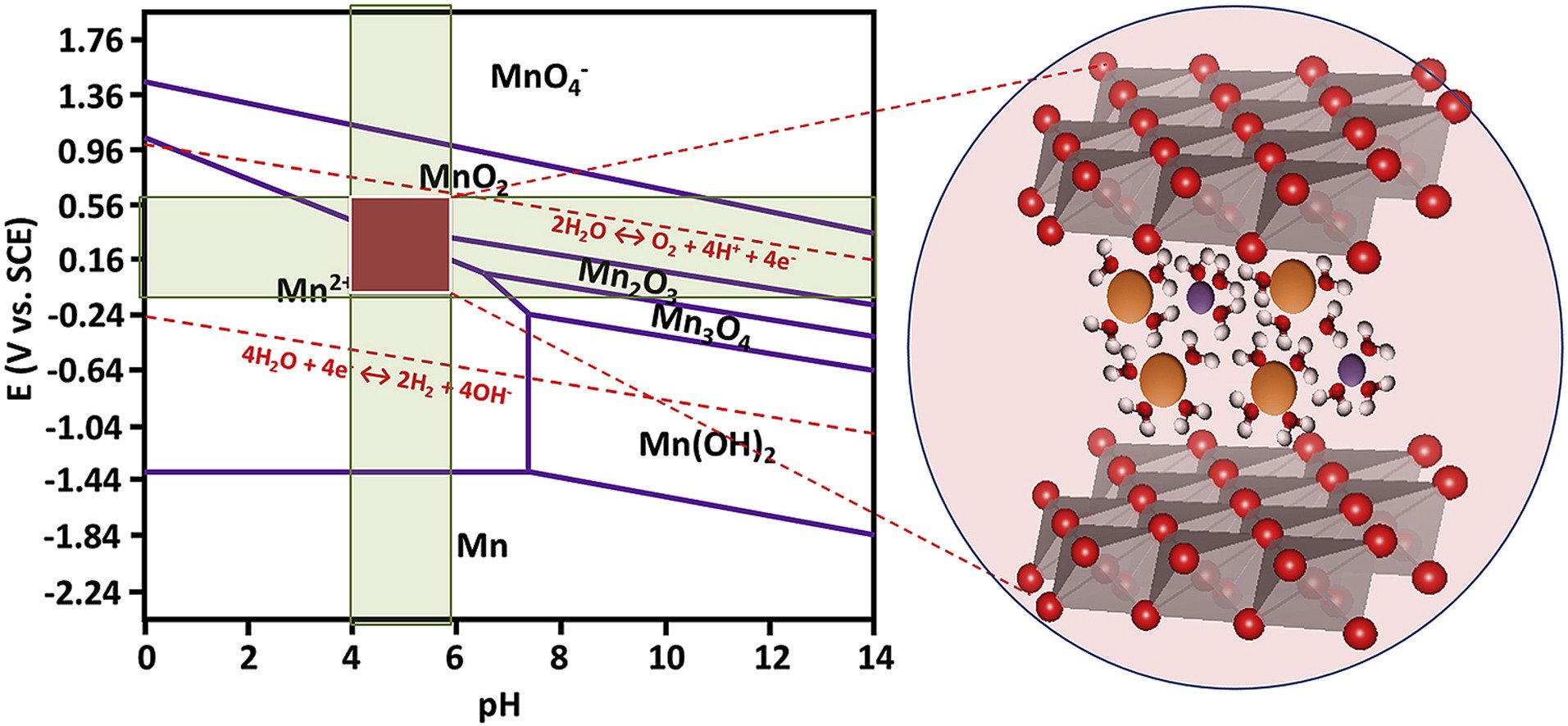

16. High-performance hybrid supercapacitor of mixed-valence manganese oxide/N-doped graphene aerogel nanoflower using an ionic liquid with a redox additive as the electrolyte: In situ electrochemical X-ray absorption spectroscopy
By: Ma, Nattapol; Phattharasupakun, Nutthaphon; Wutthiprom, Juthaporn; et al.
Electrochimica Acta, 2019, 271, 110-119
DOI: https://www.sciencedirect.com/science/article/pii/S0013468618306182?via%3Dihub
Abstract: Although electrochemical double layer capacitors (EDLCs) have high specific power, their specific energy is rather low. To address this issue, a composite material between EDLC-type N-doped graphene aerogel (N-rGOae) with an N-doped content of ca. 8 at% and pseudocapacitor-type mixed-valence manganese oxide (MnOx) nanoparticles with a diameter of <20 nm was synthesized and used as the electrode material of hybrid supercapacitors. In addition, a new electrolyte of ionic liquid electrolyte 1-butyl-1-methylpyrrolidinium dicyanamide ([BMP][DCA]) with a redox additive (K4[Fe(CN)6]) was employed to further improve the performance of the hybrid supercapacitor. The as-fabricated supercapacitor exhibits an excellent specific energy and power of 44.7 Wh kg−1 and 4551.4 W kg−1, respectively. In situ electrochemical X-ray absorption spectroscopy (XAS) was also used to investigate the charge storage mechanism of the mixed-valence MnOx in the composite. Interestingly, with the presence of redox additive in the electrolytes, a wide oxidation state variation range of Mn in the MnOx/N-rGOae composite can be observed indicating high redox activities and confirming the faradaic charge transfer interaction between redox additive and MnOx. Furthermore, the in situ XAS result also confirms the reversibility of the redox reaction of MnOx showing that the hybrid supercapacitor using the ionic liquid electrolyte with a redox additive in this work may be practically used in high-energy applications.
17. Lithium Bond Impact on Lithium Polysulfide Adsorption with Functionalized Carbon Fiber Paper Interlayers for Lithium-Sulfur Batteries
By: Maihom, Thana; Kaewruang, Siriroong; Phattharasupakun, Nutthaphon; et al.
JOURNAL OF PHYSICAL CHEMISTRY C, 2018, 122, 7033–7040
DOI: https://pubs.acs.org/doi/10.1021/acs.jpcc.7b09392
Abstract: Lithium–sulfur batteries (LSBs) are of great interest as a promising energy storage device because of their high theoretical capacity and energy density. However, they exhibit poor discharge capacity and capacity retention during long-term cycling because of their inherent drawbacks including the poor conductivity of sulfur and lithium sulfide, the shuttle effect of lithium polysulfides (LiPSs), and the large volume expansion of sulfur to lithium sulfide. An effective approach that can solve these problems is to use an interlayer inserted between the separator and the cathode. Nevertheless, the underlying adsorption mechanism of LiPSs on the interlayer has not yet been widely investigated. Herein, the effect of lithium bond chemistry on the adsorption of LiPSs on the functionalized carbon fiber paper (CFP) interlayer containing hydroxyl, carboxyl, or amide functional groups is investigated by a density functional theory approach. It is found that the functionalized CFP exhibits a strong lithium bond interaction between the Li electron acceptor of LiPSs and the N or O electron donor of the functionalized CFP interlayer. In addition, the correlation between the adsorption energy of LiPSs on the interlayer and the electrochemical performance of LSBs is investigated. The results provide the fundamental understanding of the structure–property relationship for the adsorption of LiPSs on the functional groups of the interlayer, which will be beneficial for the further development of advanced LSBs.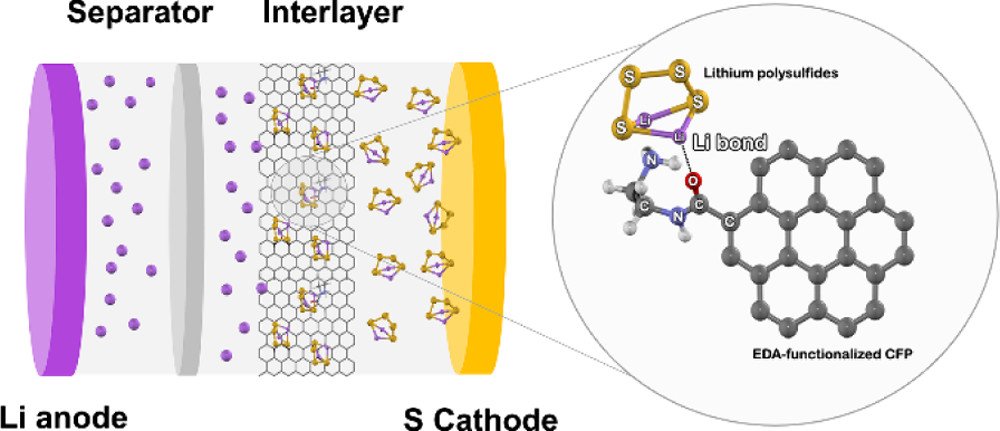

18. A computational study of the catalytic aerobic epoxidation of propylene over the coordinatively unsaturated metal-organic framework Fe-3(btc)(2): formation of propylene oxide and competing reactions
By: Maihom, Thana; Sawangphruk, Montree; Probst, Michael; et al.
Physical Chemistry Chemical Physics, 2018, 20, 6726-673
DOI: https://pubs.rsc.org/en/content/articlelanding/2018/CP/C7CP07550B#!divAbstract
Abstract: The aerobic epoxidation of propylene over the metal–organic framework Fe3(btc)2 (btc = 1,3,5-benzentricarboxylate) as catalyst has been investigated by means of density functional calculations. The mechanisms of the reaction towards propylene oxide, carbonylic products (acetone and propanal) and a pi-allyl radical were investigated to assess the efficiency of Fe3(btc)2 for the selective formation of propylene oxide. Propylene oxide and carbonylic products are formed on Fe3(btc)2 by proceeding via propyleneoxy intermediates in the first step. Subsequently, the intermediates can then either be transformed to propylene oxide by way of ring closure of the intermediate or to the carbonylic compounds of propanal and acetone via 1,2-hydride shift. The results show that the formation of propylene oxide is favored over the formation of carbonylic products mainly due to the activation barriers being 2–3 times smaller. The activation barriers for the formation of the propyleneoxy intermediates on the Fe3(btc)2 catalyst for the first and second reaction cycle are also lower than the barriers obtained for the formation of the pi-allyl radical that acts as the precursor to combustion products. On the basis of these computational results, we therefore expect a high catalytic selectivity of the Fe3(btc)2 catalyst with respect to the formation of propylene oxide. We also compared the catalytic activities of Fe3(btc)2 and Cu3(btc)2. The activation energy of the rate-determining step is almost 2 times lower for Fe3(btc)2 than that for Cu3(btc)2, due to a larger charge transfer from the catalytic site to the O2 molecule in the case of Fe3(btc)2.
19. The solution phase aggregation of graphene nanoplates
By: Krittayavathananon, Atiweena; Li, Xiuting; Sokolov, Stanislav V.; et al.
APPLIED MATERIALS TODAY, 2018, 10, 122-126
DOI: https://www.sciencedirect.com/science/article/pii/S235294071730447X?via%3Dihub
Abstract: The occurrence of particle aggregation, especially for two-dimensional (2D) nanoparticles, in the solution phase is an important practical problem. Aggregation influences the materials’ properties and behavior. This work studies the re-stacking process of graphene nanoplates (GNPs) in colloids and suspensions by a simple and highly sensitive technique in which the current responses resulting from the impacts of individual and aggregated particles which bridge across two interdigitated gold microbands are detected. The magnitude of the steps in current varies as a function of time and yield clear in situ information regarding the formation of GNP aggregation.
20. A new energy conversion and storage device of cobalt oxide nanosheets
By: Kalasina, Saran; Phattharasupakun, Nutthaphon; Sawangphruk, Montree
Journal of Materials Chemistry A, 2018, 6, 36-40
DOI: https://pubs.rsc.org/en/content/articlelanding/2018/TA/C7TA08736E#!divAbstract
Abstract: A new design of a single hybrid energy conversion and storage cell of cobalt oxide (Co3O4) nanosheets, which can absorb the whole visible spectrum, exhibits the highest volumetric capacitance of 80.8 F cm−3 under light illumination (white LED), which is 2.2-fold higher than that under dark conditions at 1.5 A cm−3.
21. High-Performance Supercapacitors of N-Doped Graphene Aerogel and Its Nanocomposites with Manganese Oxide and Polyaniline
By: Phattharasupakun, Nutthaphon; Wutthiprom, Juthaporn; Ma, Nattapol; et al.
Journal of The Electrochemical Society, 2018, 165, A1430-A1439
DOI: https://iopscience.iop.org/article/10.1149/2.0981807jes
Abstract: Nitrogen-doped graphene aerogel (NGae) with a three-dimensional (3D) interconnected structure with ca. 3.5 at% of nitrogen content was synthesized by a hydrothermal reduction of graphene oxide with hydrazine and a following freezing-dry method. The 3D morphology of conductive NGae provides fast ion diffusion leading to nearly steady specific capacitances (ca. 450 F g−1) when increasing scan rates (10–100 mV−1) or applied currents (0.5–2.5 A g−1). Not only can the nitrogen-containing groups improve the electrical conductivity of NGae but also they can store charges via their reversible surface redox reactions. The as-fabricated symmetric supercapacitor of NGae in 1 M H2SO4 electrolyte provides a maximum specific energy and power of 42 Wh kg−1 and 2077 W kg−1, respectively. In addition, incorporating pseudocapacitor materials i.e., MnO2 and polyaniline to NGae structure can also provide higher specific energy owing to surface redox reactions. The as-fabricated supercapacitors in this work may be practically used in high energy and power applications.
22. Enhancing the Charge Storage Capacity of Lithium-Ion Capacitors Using Nitrogen-Doped Reduced Graphene Oxide Aerogel as a Negative Electrode: A Hydrodynamic Rotating Disk Electrode Investigation
By: Phattharasupakun, Nutthaphon; Wutthiprom, Juthaporn; Suktha, Phansiri; et al.
Journal of The Electrochemical Society, 2018, 165, A609-A617
DOI: https://iopscience.iop.org/article/10.1149/2.0961803jes
Abstract: An energy storage device having both high energy and power density is crucial for many applications such as electric vehicles and mobile phones. Herein, pre-lithiated three-dimensional (3D) nitrogen-doped reduced graphene oxide aerogel (N-rGO aerogel) was introduced as a negative electrode to overcome the kinetic sluggish of lithium ions and activated carbon (AC) was used as a positive electrode of lithium-ion capacitor (LIC). The electrochemical property of electrode materials was studied in a three-electrode system using a hydrodynamic rotating disk electrode at different rotation rates confirming the lithium ion chemisorption on the N-rGO aerogel. The as-fabricated LIC tested in a cell voltage window of 2.0–4.0 V can provide a maximum specific energy of 170.28 Wh kg−1 (144.79 Wh L−1), a maximum specific power of 25.75 kW kg−1 (21.89 kW L−1) with ~100% capacity retention and 100% coulombic efficiency over 2000 cycles. The excellent performance stems from the nitrogen-containing group and 3D interconnected structure of the N-rGO aerogel. The LIC in this work may be used in many high energy and power applications.
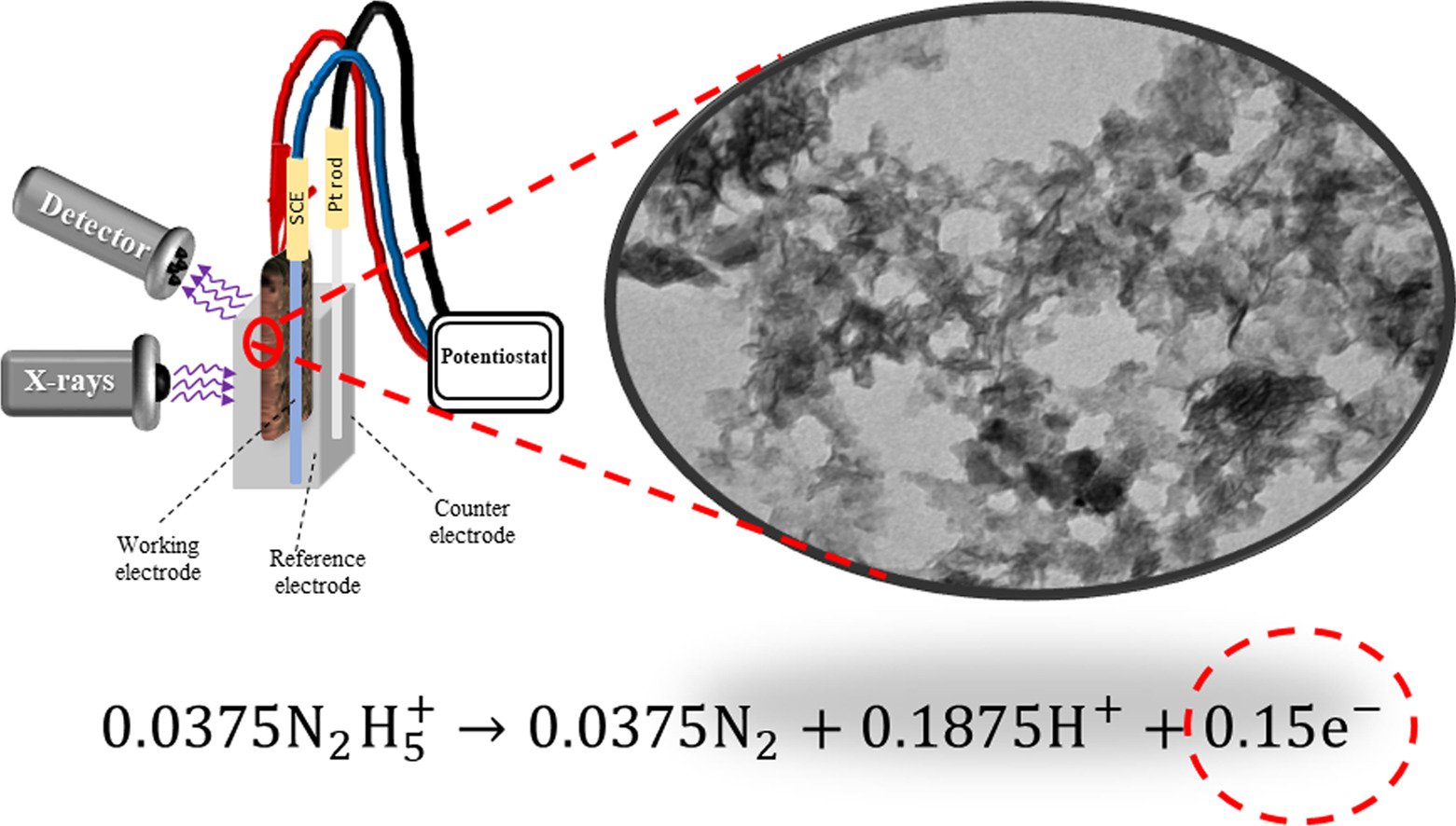
23. Layered manganese oxide nanosheets coated on N-doped graphene aerogel for hydrazine detection: Reaction mechanism investigated by in situ electrochemical X-ray absorption spectroscopy
By: Wuamprakhon, Phatsawit; Krittayavathananon, Atiweena; Ma, Nattapol; et al.
Journal of Electroanalytical Chemistry, 2018, 808, 124-132
DOI: https://www.sciencedirect.com/science/article/pii/S1572665717308780?via%3Dihub
Abstract: Understanding the sensing reaction mechanism at the solid electrode-liquid electrolyte interface during the detection is crucial for developing chemical sensors. In this work, layered MnO2 nanosheets were coated on nitrogen-doped reduced graphene oxide aerogel (3D-N-rGO) rotating disk electrode (RDE) and used towards hydrazine detection. The successive reduction in the oxidation number of Mn in the MnO2/3D-N-rGO electrode during the detection monitored by an in situ electrochemical X-ray absorption spectroscopy (XAS) indicates that 0.15 mol electrons produced from the oxidation of hydrazine do transfer to MnO2. The hydrodynamic diffusion of hydrazine from bulk solution to the surface of the MnO2/3D-N-rGO RDE investigated also plays a major role in the sensitivity of hydrazine detection. The limit of detection (LOD) value at a signal-to-noise ratio of ca. 3 is 085.0 μM (4000 rpm) with a response time of < 2 s and wide linear concentration ranges. The MnO2/3D N-rGO RDE can detect trace hydrazine without any effect of high concentration interferences (20–100 μM) including glucose, caffeine, methylamine, ethylenediamine, n-butylamine, adenine, cytosine, guanine, and l-arginine. In addition, the MnO2/3D N-rGO RDE sensor can practically be used to detect trace hydrazine in real-world samples i.e., drinking water and lake water.